Biogeography of marine actinomycetes and the secondary metabolites they produce
Issue: Biogeography
27 August 2013 article

The discovery of new antimicrobials has failed to keep up with the rise of drug-resistant bacterial infections. But might the answer come from the marine environment? And what opportunities does this provide for finding correlations between taxonomy, biogeography and secondary metabolite production?
Since the discovery of streptomycin by Selman Waksman in 1943, microbially derived antibiotics have dramatically improved human health and well-being. But not all bacteria produce antibiotics. In fact, the vast majority of microbially derived antibiotics have been discovered from the genus Streptomyces, which single-handedly played a major role in shaping the pharmaceutical industry in the mid-20th Century. Despite the importance of microbially derived natural products as a source of antibiotics and other medicines, diminishing returns on actinomycete-based drug discovery efforts have led the pharmaceutical industry away from soil microbes as a resource for new drug leads in favour of alternative discovery platforms, such as combinatorial chemistry. Unfortunately, these new approaches have not kept pace with the demands to treat drug-resistant bacterial infections and other chronic diseases. In response, there has been a resurgence of interest in natural product research that includes the exploration of new microbial resources such as those that occur in the world’s oceans.
The genus Salinispora
While many of the actinomycetes that can be cultured from marine samples appear no different from those that occur on land, there are exceptions. One example is the genus Salinispora. This taxon displays clear evidence of adaptation to life in the sea, including a failure to grow when seawater is replaced with deionised water in the growth medium. Most reports of this taxon come from marine sediments, where they have been estimated to occur in abundances of approximately 103 per ml. When compared to total bacterial counts of 109 per ml, they can be considered a part of the ‘rare biosphere’. Salinispora species are a rich source of secondary metabolites, including salinosporamide A, which has entered clinical trials for the treatment of cancer (Fig. 1). This finding has prompted extensive surveys of the diversity and distributions of Salinispora species in the hopes of finding additional drug candidates and other structurally unique secondary metabolites. The results have provided opportunities to test for correlations between Salinispora taxonomy (who they are), biogeography (where they live), and secondary metabolite production (selective traits that provide survival advantages).
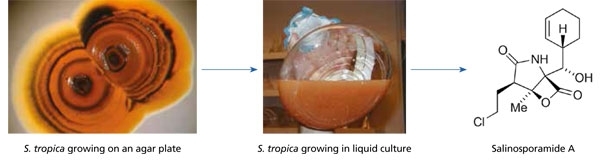
Fig. 1. Compounds derived from marine micro-organisms that are in clinical development for the treatment of cancer include salinosporamide A, which was discovered from S. tropica. This compound is a potent and highly selective inhibitor of the 20S proteosome and is being evaluated for the treatment of multiple myeloma in addition to other types of cancer.
While there are clear barriers to surviving dispersal in the case of bacteria that inhabit extreme environments such as hot springs, barriers are less apparent for actinomycetes such as the genus Salinispora, which produce resistant spores that have the capacity to remain dormant until suitable growth conditions are encountered. Thus it is not surprising that Salinispora species have been cultured from near-shore tropical and sub-tropical sites around the globe (Fig. 2). What is more surprising is that they have yet to be cultured from deeper or more temperate locations despite having been detected with culture-independent methods from these locations, including ocean sediments as deep as 3,000 m in the South Pacific Gyre and greater than 5,000 m in the Canary Basin. While the conditions required for the cultivation of these deep-sea strains remain unknown, their detection provides intriguing evidence for global oceanic dispersal.
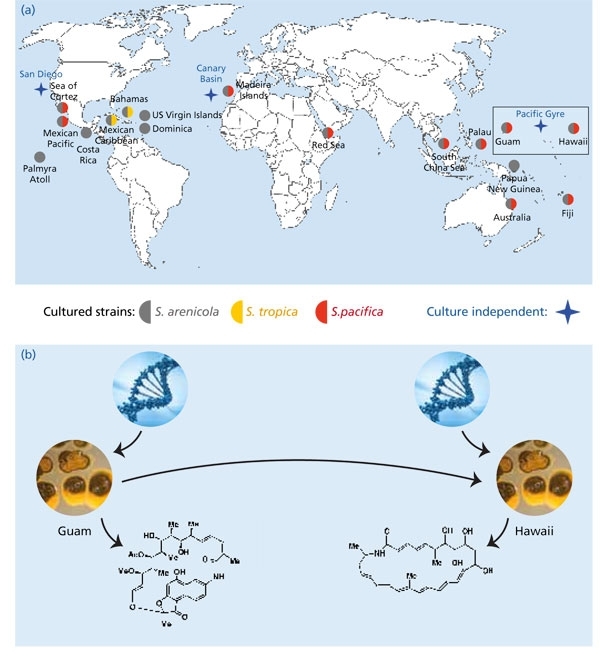
Fig. 2. Global distribution of Salinispora species. (a) Reports come from multiple research groups and global collection sites and include the culture-independent detection of the genus in sediments collected as deep as 5,000 m from the Canary Basin. (b) There is evidence that Salinispora species (pictured here as colonies growing on agar plates) are sampling secondary metabolite biosynthetic pathways from a local gene pool. The products of these pathways include biologically active small molecules, which may provide a selective advantage in that particular environment. As strains move to a new location (direction inferred), they will have access to a new gene pool that may provide opportunities to test different secondary metabolites.
At the species level, biogeographic patterns begin to emerge with S. tropica having only been reported from the Caribbean, and S. pacifica from most sites except the Caribbean. This could represent an example of speciation due to geographic isolation. However, there are other potential explanations, including environmental factors that prevent the growth of one species at a particular site or relative abundances (i.e. one species may be below the detection limits of the methods applied). While it’s relatively straightforward to demonstrate the presence of an organism at a specific site, it’s much more difficult to demonstrate its absence, as made clear by the adage ‘absence of evidence is not evidence of absence’. Deep-sequencing techniques will provide new opportunities to further test for the presence of the three Salinispora species among global collection sites and better address the Baas Becking hypothesis ‘everything is everywhere, but the environment selects’.
The biogeography of S. arenicola suggests a different evolutionary paradigm. This species has the broadest distribution, having been recovered from all sites from which the genus has been reported. Its consistent co-occurrence with the other two species suggests they may be ecologically distinct. While any such differences have yet to be clearly established, it is interesting to note that the major phenotypic trait (subsequently confirmed at the genomic level) that differentiates the three Salinispora species is secondary metabolism. This presents the intriguing possibility that secondary metabolites represent ecotype-defining traits in Salinispora species. This possibility is all the more surprising given that the genes responsible for secondary metabolite biosynthesis in these bacteria are known to be subject to horizontal gene transfer.
It is now well established that horizontal gene transfer is a major force in bacterial evolution. This is a remarkably effective process that provides the immediate opportunity to test the products of new genes as opposed to waiting for existing genes to evolve. In the case of secondary metabolism, it’s not only a single gene that can be transferred, but also entire gene collectives that account for secondary metabolite assembly and also regulation, transport and resistance. The transfer of these pathways, which can exceed 100 kb, provides immediate opportunities to test the effects of new secondary metabolites on fitness.
The biogeography of Salinispora secondary metabolism is providing interesting insight into the ecology and evolution of the three species. One of the more interesting observations is that all S. arenicola strains examined to date, including those derived from global collection sites, produce compounds in the rifamycin class. There is clear evidence that the biosynthetic pathway responsible for the production of this compound was acquired by horizontal gene transfer, yet it has become a diagnostic phenotype of the species. The notion of a chemotaxonomy associated with secondary metabolism is at odds with horizontal gene transfer, and implies the existence of strong selective pressures to maintain the production of this compound after the pathway was acquired. Given that S. arenicola is essentially a clonal species at the 16S rRNA level, the population that acquired this pathway has spread via ocean currents at a pace that far exceeds the rates at which changes are accumulated in this gene.
While it is clear that some pathways can be shared among all individuals within a Salinispora species, the distributions of most pathways are more limited. In particular, there is evidence that pathway distributions are correlated with location, suggesting that populations are sampling from local gene pools. On might imagine that Salinispora species are constantly acquiring new biosynthetic pathways and testing their products in an ongoing experiment in chemical ecology and natural selection (Fig. 2). In some cases, the pathways sweep through the entire population such that the trait becomes a phenotypic marker for the species. Alternatively, the acquisition of a pathway may provide new opportunities for niche exploration and thereby drive diversification. If correct, these processes imply that secondary metabolism may be far more important for the ecology and evolution of bacteria than previously appreciated.
Conclusions
We are entering a new age of natural product discovery that is being guided by genomic blueprints and bioinformatic assessments of secondary metabolite biosynthetic potential. Yet the genetic resources that will inform future discoveries remain distributed among strains and habitats in ways that we have not yet begun to decipher. The emergence of genomics-based natural product discovery will inevitably provide exciting new insight into the biogeography of natural-product-producing bacteria and the biosynthetic genes that assemble these useful compounds.
Paul R. Jensen
Scripps Institution of Oceanography, University of California San Diego. Email: [email protected].
Acknowledgements
The author acknowledges financial support from the National Institutes of Health (NIH), Fogerty Center International Cooperative Biodiversity Groups program (grant U01- TW007401-01).
Image: Fig. 1. images by Paul R Jensen. Fig 2. graphics by Pauline Capote. DNA image from iStockphoto/Thinkstock..


