The integration of nanoscale imaging and quantitation of nanomechanical properties in microbiology
Issue: Future Tech
09 August 2016 article
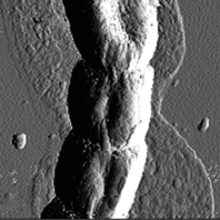
Recent developments in nanotechnology enable the imaging, quantification and manipulation of materials at the near-atomic level. The number of applications of atomic-force microscopy (AFM) in the life sciences is increasing, now allowing the integrated study of topological and quantitative nanoscale mechanical characterization of living cells and their interactions with their environments, which was inconceivable until recently.
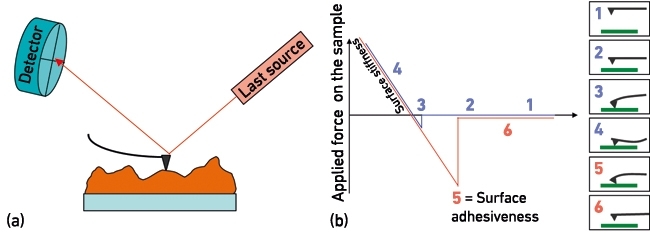
AFM is a surface-sensitive technique whereby a sample is scanned with a sharp tip mounted on a micrometre-sized flexible cantilever. Irregularities in surface topography will deflect the cantilever while scanning the surface (Fig. 1a). These deflections are detected, processed and then used to reconstruct a 3D topological image of the sample. Forces between the surface and cantilever tip govern the cantilever deflections differentially when moving towards or away from the sample (Fig. 1b), from which a range of mechanical properties can be quantified at the nanoscale, such as surface stiffness, adhesion and deformation.
Individual microbial cells, cells within a microbial community, and their subcellular components can readily be imaged by well-known techniques such as confocal laser scanning microscopy (CSLM) and scanning electron microscopy (SEM). These techniques require staining, labelling and/or other sample preparations, which may introduce artefacts. Most excitingly, microbial materials can be analysed quantitatively by AFM under physiological conditions without the need for prior sample preparation.
FIG. 1. BASIC WORKING PRINCIPLES OF AFM: CANTILEVER DEFLECTIONS LEADING TO DETERMINATION OF (A) SURFACE TOPOGRAPHY, AND (B) FORCE-CURVE MEASUREMENTSE FOR QUANTIFICATION OF NANOMECHANICAL PROPERTIES.
Nanomechanical properties of microbial cell surfaces and extracellular (polymeric) substances in pure cultures and biofilms
Extracellular polymeric substances (EPS) form a composite biomaterial of water, proteins, nucleic acids and polysaccharides in which microbial cells are embedded to construct a biofilm that favours microbial growth and survival by permitting the local accumulation of nutrients and other metabolites. Biofilms form the main method of microbial colonisation of natural and artificial substrates, with potential to cause significant risk to health and the economy, e.g. by biofouling medical and industrial materials such as implants and membranes. Studying the properties of biofilms and their development is therefore of paramount importance, not only for the advancement of microbiology in general, but also to control detrimental microbial effects on specific applications.
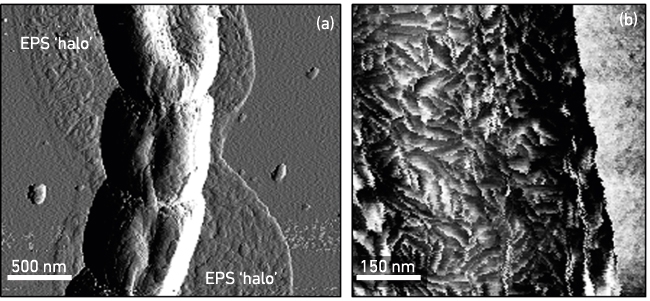
High-resolution topographical imaging and mechanical force analysis of composite biomaterials in their natural state by AFM has already demonstrated its usefulness in studying different stages of biofilm and other types of multicellular development. AFM has revealed that cells are first surrounded by a thin ‘halo’ of secreted EPS (Fig. 2a), which can be up to a cell’s diameter away from the cell surface and a few tens of nanometre in thickness. Importantly, the microbial community can produce overlapping EPS layers with different mechanical properties. Interestingly, both filamentous bacteria (Fig. 2a) and fungi secrete EPS in a similar way, showing that organisms from different kingdoms with similar cellular morphology (growing by hyphal elongation from the tip) can colonise and affect surfaces in a similar way. The next stage of cellular differentiation can also be followed with AFM: chaplin and rodlin proteins are excreted on the aerial cell surface of Streptomyces bacteria, which then typically self-assemble into thicker rodlet-shaped amyloidal aggregates, conferring a concomitant change in mechanical properties from wettable to water-repellent (Fig. 2b). This change in surface properties can be visualised and quantified with AFM, providing quantitative nanomechanical insights into morphological differentiation.
AFM can also be co-applied with other analytical techniques such as CSLM, nanoscale secondary ion mass spectrometry (NanoSIMS), and several others. Such hybrid instrumentation can reveal subcellular localisation of labelled intra- and extracellular molecules, with overlying bionanomechanical properties, leading to deep mechanistic insights such as those demonstrated for the lateral expansion of antimicrobial pores in lipid bilayers.
FIG. 2. TOPOLOGY OF A FILAMENTOUS BACTERIAL HYPHA SURROUNDED BY A THIN FILM OF EPS (A) AND RODLET-STRUCTURED AERIAL CELL SURFACE AT HIGHER MAGNIFICATION (B).
Nanoscale detection of microbial processes in situ: bio-weathering of minerals
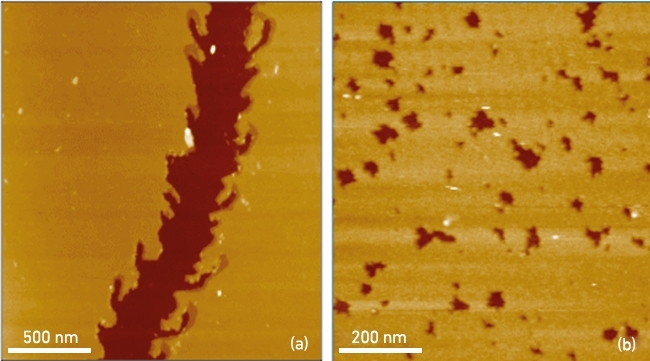
Terrestrial microbes play a pivotal role in mineral weathering, soil formation and the generation of essential growth nutrients. This process is especially evident in forested ecosystems dominated by ectomycorrhizal symbioses with tree roots. The role of these fungi in mineral weathering can be assessed in situ by evaluating the presence of microbial signatures in rocks and soil particles, such as etch pits, secondary mineral formation and hyphal-shaped channels (Fig. 3a). Inductively-coupled plasma atomic emission spectroscopy (ICP-AES) can be applied to give the overall dissolution rate of minerals, e.g. released mineral cations in solution at parts-per-billion (ppb) levels. AFM can complement ICP-AES data by detecting in real time the release of mineral components, the specific geometry of bioweathering features, and the different reactivity of mineral surfaces resulting from exposure to fungal exuded substances such as organic acids (Fig. 3b). Furthermore, the specific nanoscale geometries of such microbial signatures alongside AFM characterisation of mineral components refractive to dissolution have also provided a novel molecular mechanism for mineral bond hydrolysis and dissolution.
FIG. 3. BIOWEATHERING OF SILICATE MINERALS BY MICROBIAL SIGNATURES: (A) FUNGAL HYPHA-CREATED CHANNEL AND (B) ETCH PITS RESULTING FROM FUNGAL ORGANIC ACID EXUDATES.
Nanoscale detection of microbial processes in situ: soil
Microbial activities greatly affect soil properties and soil ecosystem processes such as the formation and breakdown of soil organic matter (SOM). Different techniques exist to evaluate macroscale soil properties such as composition, wettability, bulk density, porosity, soil hardness and deformation. In turn, these properties may affect the severity and impact of runoff, flood risk, soil stability to tillage, and other agricultural and anthropogenic activities. The application of AFM to soils allows nanoscale imaging and quantification of the nanophysical properties of soil building blocks, i.e. single particles and soil aggregates, to understand how these ultimately influence bulk soil properties.
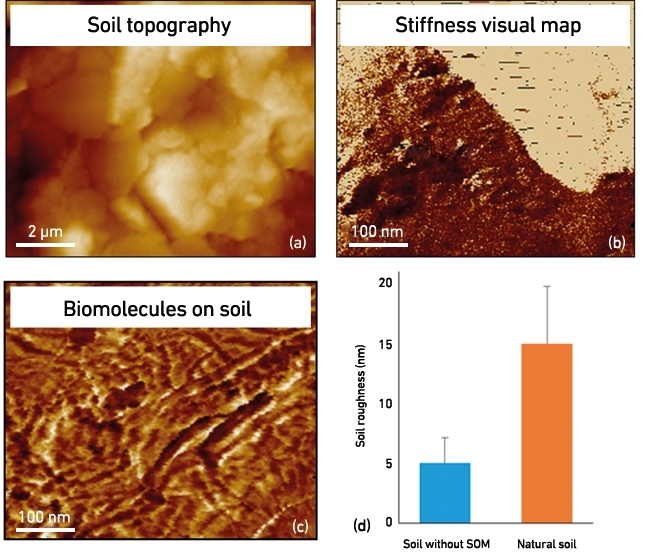
For example, the presence of SOM strongly affects the physical properties of soil aggregates. By applying AFM, high-resolution topological maps can be obtained for mineral surfaces of soil particles and the presence, distribution and nature of biological and other organic matter that is covering soil minerals (Fig. 4). How the nanomechanical properties are affected by SOM can be measured, for instance, by the change in elastic modulus (Young’s modulus) in the presence and absence of microbial or plant exudates (Fig. 4b), while the surface roughness of single aggregates appears to increase with the presence of bio-organic matter (Fig. 4d). It is therefore very timely to integrate nanoscopic profiling and bulk soil characterisations with functional expression studies, such as metaproteomics, to generate deep mechanistic understanding of the role of microbes in soil ecosystems.
FIG. 4. NANOSCALE NATURAL SOIL CHARACTERISATIONS: (A) TOPOGRAPHY OF SOIL PARTICLE, (B) MINERAL SURFACE WITH DIFFERENT STIFFNESS PROPERTIES (UNIT MPA), (C) BIOMOLECULES ON A SOIL PARTICLE, (D) QUANTITATIVE EXEMPLAR OF THE NANO-ROUGHNESS OF NATURAL AND ORGANIC MATTER-DEPRIVED SOILS.
Microbiology: crossing scales
Exciting novel microbial properties and behaviours can be revealed by applying nanoscale analytical techniques per se to pure cultures and in situ ecosystems. The combined application of biomolecular, nanoscale and macroscopic techniques has the potential to stepwise fill the gaps in understanding microbial(-influenced) processes in the real world across molecular to global scales.
S. ANDREA GAZZE, LEWIS FRANCIS & GEERTJE VAN KEULEN
Institute of Life Science, Swansea University, Swansea SA2 8PP, Wales, UK
[email protected]
[email protected]
[email protected]
FURTHER READING
Gazze, S. A. & others (2013). Nanoscale observations of extracellular polymeric substances deposition on phyllosilicates by an ectomycorrhizal fungus. Geomicrobiol J 30, 721–730.
Gazze, S. A. & others (2014). Chlorite topography and dissolution of the interlayer studied with atomic force microscopy. Am Mineral 99, 128–138.
Matthews, G. P. & others (2016). Soil hydrophobicity – relating effects at atomic, molecular, core and national scales. Geophys Res Abstr 18, 8478.
Rakowska, P. D. & others (2013). Nanoscale imaging reveals laterally expanding antimicrobial pores in lipid bilayers. Proc Natl Acad Sci U S A 110, 8918–8923.
Image: Figs. 1, 2, 3 and 4. S. Andrea Gazze..


