Comment - MERS-Coronavirus: occasional zoonotic or emerging pathogen?
27 August 2013
Following the first reports of MERS-CoV in 2012 in Saudi Arabia, and the media frenzy following the appearance of cases in Europe, what do we know about this novel virus and its potential to develop into a serious threat to public health?
A new coronavirus causing acute respiratory distress syndrome was first reported in Saudi Arabia by Ali Mohamed Zaki in a ProMED posting in 20121. The virus was characterised at Erasmus Medical Center in Amsterdam resulting in the name new coronavirus-EMC, nCoV-EMC. More recently, the name of Middle Eastern Respiratory Syndrome coronavirus, MERS-CoV, has been suggested to reflect the predominant geographical location of cases to date (Fig. 1)2. Infection can give rise to severe respiratory distress and of the 91 cases documented by the World Health Organization (WHO) since September 2012, 46 have died, making the mortality rate as high as that of H5N1 influenza and higher than that of the SARS-CoV outbreak of 2003. Although mostly confined to the Middle East, interest in Europe has been sparked by people returning with the infection after visiting the region and by patients from elsewhere being flown in for expert medical care. Person-to-person transmission, first reported in a hospital in France and associated with a minor media frenzy (Fig. 2), is now confirmed in other cases3, albeit predominantly in a hospital setting. The obvious questions are: how do individuals become infected in the first place, and what level of threat does the new virus represent? Are these unusual sporadic cases, or are we witnessing the emergence of a new virus destined to spread among humankind? To date these questions have been difficult to answer with any certainty.
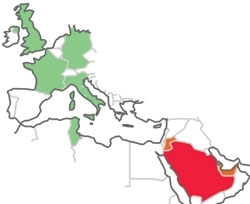
Fig. 1. Cases of MERS-CoV to date have centred on Saudi Arabia. Cases in the countries shown are limited (one or two) and have been in people who have either directly returned from the Gulf or have had close contact with those already infected.
The MERS-CoV virus is most closely related to HKU4 and HKU5, both coronaviruses of bats2,4. Aside from SARS-CoV, which no longer circulates in man, there are four extant co-circulating human coronaviruses. Serological evidence suggests that most people have been infected by all of them by their teens. For the most part they cause mild respiratory disease, although more serious clinical cases can occur, often with other underlying infections. The relationship of MERS-CoV with bat viruses echoes the findings for SARS-CoV that originated in the Chinese horseshoe bat5. Indeed bats have been found to harbour a large number of viruses, their dense colonies providing ideal conditions for virus circulation, and they may be the original source of many coronaviruses6. If bats and man interacted more extensively we would probably see a number of new zoonotic infections, but as bat roosts are generally remote, direct infection of people from bats is a rarity, which brings us back to the question of MERS-CoV and its origins. The low number of cases means a common link to a source of infection has not emerged. Animals common to the Middle East, such as goats and camels, have been suggested2 , and recently evidence for previous infection of dromedary camels has been reported10. It is unclear if this could act as the animal reservoir as camel herders and farmers have not been among the infected. A poorly transmissible but distributed source of infection might also fit the current pattern, for example, infection via a food substance, perhaps a regional food. The bat link emerges again here – just as the WHO reported human-to-human transmission of MERS-CoV, another bat virus, Nipah virus, caused 21 deaths in Bangladesh7. Nipah, a paramyxovirus, has been known since 1998 and causes regional small-scale outbreaks of human infection, also with a high fatality rate. The virus is transmitted by bat saliva and urine contamination of foodstuffs, date palm sap in the case of the current Bangladeshi outbreak. Something similar could be the case for MERS-CoV. The Nipah outbreak is less reported because it is not a new virus and, since the SARS episode, there is a spook factor associated with the name coronavirus. No one would wish to underplay the seriousness of MERS-CoV infection – it kills a large proportion of those it infects, and the opportunity for mutation in this largest of RNA virus genomes means that adaptation is certainly possible. But at the moment there is little evidence to suggest MERS-CoV is anything more than another Nipah-like occasional zoonotic that is unlikely to spread on a wider scale.
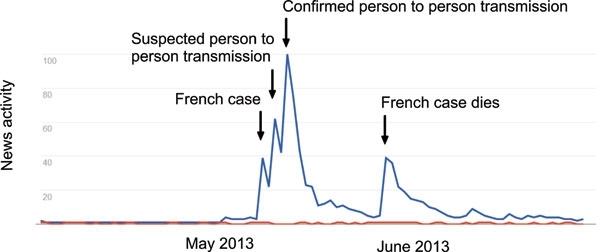
Fig. 2. Unbalanced media attention? A Google trends output of ‘Coronavirus’ (blue) and ‘Nipah’ (red) for 90 days in May and June 2013. The coverage of the MERS-CoV cases in Europe may be understandable, but it ignores similar infections elsewhere.
Of the infection itself, a lot remains to be learned. In SARS, virus attachment to the cellular receptor ACE2 causes receptor shedding that exacerbates the pathology8. The MERS-CoV receptor has been identified as CD269, but there is little data yet on whether receptor impairment has a role in the outcome of infection. It is curious that despite being caused by different viruses, the mortality rate for MERS-CoV or Nipah, or influenza H7N9 come to that, is similar, suggesting that the pathological mechanisms triggered are perhaps shared, plausibly the cytokine storm leading to lung damage and a rapid decline or slow recovery. There are neither effective drugs nor vaccines so patient support is the only clinical treatment option. As cases accumulate, passive immunotherapy with convalescent serum from survivors might be a possibility, but whether the logistics required to achieve this are feasible is unclear. As with any zoonotic of this type, surveillance and as rapid a diagnosis as possible, offer the best chance of a recovery outcome.
Judging the likely threat of any new pathogen with so few cases and a general lack of data is only marginally better than guesswork. But based on the facts to date, the occasional zoonotic pattern seems to me to be the most likely outcome in the case of MERS-CoV. I hope I am not wrong.
Ian Jones
University of Reading. Email: [email protected].
References
- Zaki, A.M. (2012). Novel coronavirus – Saudi Arabia: human isolate. Int Soc Infect Dis.
- de Groot, R.J. & others (2013). Middle East Respiratory Syndrome Coronavirus (MERS-CoV); Announcement of the Coronavirus Study Group. J Virol 87, 7790–7792.
- Assiri, A. & others (2013). Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N Engl J Med.
- Zaki, A.M. & others (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med, 367 1814–1820.
- Lau, S.K. & others (2005). Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A 102, 14040–14045.
- Woo, P.C. & others (2009). Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med (Maywood) 234, 1117–1127.
- Institute of Epidemiology (2013). D.C.a.R. Nipah Infection in 2013 (cited 2013 30/06/2013).
- Glowacka, I. & others (2010). Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol 84, 1198–1205.
- Raj, V.S. & others (2013). Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495, 251–254.
- Reusken, C.B.E.M. & others (2013), Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis.


