Known-knowns, known-unknowns and unknown-unknowns: the mysteries of Chlamydia
21 May 2013
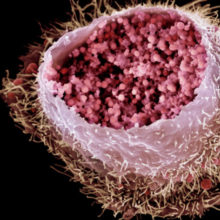
The difficulty in growing and handling Chlamydia in the laboratory has always hampered study of this important bacterium. However, recently genomics studies have allowed re-assessment and re-interpretation of what we thought we knew.
NICHOLAS R. THOMSON
Chlamydia trachomatis is an important and fascinating bacterium from many viewpoints. It is well known that chlamydia is the most prevalent bacterial sexually transmitted infection (STI) in the world, and in the UK costs the NHS up to £100 million every year (www.chlamydiascreening.nhs.uk). It also belongs to a bacterial order whose members exhibit some unique biology, which has been uncovered by exquisite experiments using cell culture, microscopy and immunohistochemistry.
It is the obligate intracellular nature and difficulty in growing and isolating this organism that has presented one of the main challenges of working with Chlamydia.
Despite its importance, until recently we have understood little about the natural history and evolution of this organism – a fact that is starting to be addressed through whole-genome sequence analysis. The application of new sequencing technologies has provided evidence that many of the established dogmas that have influenced a generation of researchers and epidemiologists alike, such as the notion that C. trachomatis does not recombine, turn out to have been based on unsafe assumptions or are simply wrong. This article will provide an introduction to the organism and the disease and will focus on recent insights into the biology of C. trachomatis, made possible by the falling costs of DNA sequencing.
What we now know about the bacterium
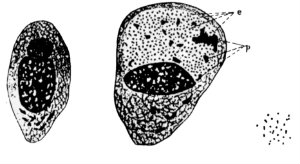
C. trachomatis was first described in 1907 by Halberstaedter and von Prowazek who presented beautiful hand-drawn figures showing infected stained conjunctival epithelial cells with ‘intracytoplasmic vacuoles’ containing small and large particles. These sacks filled with C. trachomatis cells are now known as inclusions and the small and large particles are the two forms of Chlamydia cells, known as the elementary body (EB) and reticulate body (RB), respectively (Fig. 1). These cell types and structures are part of the distinct biphasic developmental cycle exhibited by all members of the order Chlamydiales. In brief, the infectious, but non-replicative, EBs bind to the host cell and are endocytosed. Once internalised, they are sequestered into specialised vesicles, the inclusions, where the EBs differentiate into larger, metabolically active and replicative RBs. After about 2 days, the RBs begin to revert back to EBs before being released into the milieu following host cell lysis where the infectious cycle continues. It is largely because of this complex developmental cycle that the true nature of Chlamydia has remained such a mystery for so long. From the early 1900s until as late as the 1960s, Chlamydia was thought to be a virus because it could not be grown in artificial medium and because the EBs are so small (0.3 μm) that they can pass through bacterial filters. The then known ‘trachoma virus’ was first isolated in 1957 having been grown in embryonated chicken eggs and Koch postulates were fulfilled by 1958, but only in the late 1960s was it clear that the causative agent was a bacterium (see www.chlamydiae.com for an outstanding account of chlamydial biology). It is the obligate intracellular nature and difficulty in growing and isolating this organism that has presented one of the main challenges of working with Chlamydia. In equal measure, the lack of a system with which to transform this bacterium has considerably hampered research, meaning that there are no molecular tools with which to study gene function; however, this is beginning to change.
How does C. trachomatis impact on human health?
There are two biovars of C. trachomatis. The first, the trachoma biovar, is characterised by causing localised infections of the epithelial surface. This biovar includes the urogenital strains that cause the majority of STIs (there were an estimated 101.5 million new cases among adults in 2005): clinical manifestations range from long-term asymptomatic infections to complications including pelvic inflammatory disease, epithelial scarring and infertility in women and epididymitis in men. Ocular strains also belong to the trachoma biovar and are the leading cause of infectious blindness world-wide with >40 million people thought to have active disease. The second biovar, Lymphogranuloma venereum (LGV), is distinguished by the ability to spread systemically and cause swelling of lymph glands, characteristic of a bubonic disease. Currently, these biovars are subtyped based on the sequence of the ompA gene, encoding the primary surface antigen. Based on ompA-typing, C. trachomatis can be subdivided into >15 genotypes: the ocular strains include genotypes A to C, the urogenital strains include genotypes D to K, and LGV includes genotypes L1, L2 and L3 and the new variants L2b and L2c.
What have we found through genomics?
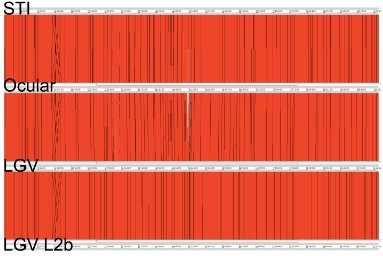
By comparing the genomes of single ocular, urogenital and LGV strains, it was apparent that C. trachomatis has a small conserved genome of around 1.1Mb, containing approximately 900 genes, which shares a high level of synteny and sequence conservation between strains and has very few ‘whole-gene’ differences. It is clear from its genome that the species has followed a reductive evolutionary path, losing functions that are likely to have been important for growth outside of the host. There have been new discoveries, such as a Type III secretion system and the presence of the machinery necessary for recombination, which was surprising for a bacterium that was once thought not to recombine. More fine-scale differences between the urogenital and ocular strains include the possession of a functional cytotoxin remnant and an intact tryptophan synthetase gene, both of which have been used to explain differences in preferred infection site. Despite the clear differences in disease outcome, in general, the contents and architecture of the genome are highly conserved at this level between ocular, urogenital and LGV strains, leaving no obvious smoking gun but some subtle differences (Fig. 2).
What we know about chlamydial populations
To provide the power to interpret the fine-scale differences between biovars, bacterial genomics has recently turned towards large-scale population-based sequencing projects. In the past 2 years, more than 58 genomes of all genotypes have been published by us and others. This number is small compared to sequencing studies of many other bacteria, reflecting the difficulty in growing and handling this organism. Despite this, these new data rephrased what we thought we knew about C. trachomatis.
The whole-genome phylogeny now provides a robust scaffold upon which to frame C. trachomatis biology.
In our work, we looked at a global panel of strains representing all disease-causing C. trachomatis variants. We showed that LGV strains form a separate clade exhibiting low diversity, with the urogenital strains split into two distinct clades, T1 and T2 (T stands for trachoma), with T1 also including the ocular strains. Over the last few years, it had become clear that the dogma relating to Chlamydia not recombining may have been an unsafe assumption. From our analysis, there was clear evidence of widespread DNA exchange between clinical isolates affecting different body sites and causing both sexually transmitted and ocular infections. This is exemplified by the ompA gene itself, which we showed was a chimera formed by a ‘mix and match’ process of recombination and exchange, whole or in part, between strains.
Why is this important? The whole-genome phylogeny now provides a robust scaffold upon which to frame C. trachomatis biology. It now is clear that we knew little about the true diversity of the circulating Chlamydia, as it was effectively being masked by the standard typing methodology. From this phylogenetic framework it is now relatively straightforward to spot genetic regions that have been exchanged and so it’s clear that co-infection with two different types of C. trachomatis may not be as rare as previously thought. Following on from this, the barriers that we assumed would prevent recombination, such as Chlamydia’s closeted intracellular lifestyle, and a strong association between genotype and site of infection, appear not to exist, at least in the way we previously thought. Together, these data have major implications for our understanding of Chlamydia epidemiology.
What we know, don’t know and thought we knew about chlamydia epidemiology
Based on traditional assumptions that the ompA genotype was a reliable predictor of relatedness, studies looking at urogenital strains have shown that the most common genotypes world-wide are E, F and D. This has led to a pervasive notion that the overall distribution of genotypes is stable, i.e. chlamydia lacks the dynamism displayed by most infectious diseases; this makes little sense. Moreover, using ompA-typing there is almost an equal number of studies that have shown an association when looking for links between genotype and the hosts’ age, gender, number of sexual partners or clinical symptoms, compared to those that have not. If ompA-genotypes occupy unrelated positions on the phylogenetic tree due to recombination, this could explain the disparity between studies. The good news is that by having a detailed population framework for C. trachomatis, we now have the tools with which to carry out this sort of analysis and make these associations, should they exist.
One salient tale about the dynamism of C. trachomatis comes from Sweden, where, in 2006, some counties noticed a drop in chlamydia cases. A new variant of C. trachomatis (nvCT) had emerged that was evading several commercial molecular diagnostic tests based on the presence of specific plasmid sequences. A 377bp deletion in the plasmid removed the test target, meaning nvCT strains returned a negative diagnosis when a single target test was applied. Despite the apparent drop in incidence, the truth was that failure to detect, and hence to treat those infected with the new variant, had led to a significant increase in cases. In some Swedish counties 20–64% of current infections are now caused by nvCT. A case of Darwinian diagnostic-driven evolution provides the opportunity for the nvCT to evade the test and be promoted to dominance in a short period of time.
What’s new? Lots!
Technologically there have been two recent breakthroughs. The first was by a group in Southampton lead by Yibing Wang and Ian Clarke who have shown for the first time that Chlamydia is transformable. Using a simple calcium-chloride-based approach, they successfully introduced a GFP-tagged plasmid into a plasmid-free C. trachomatis strain, opening up one of the most significant barriers for chlamydial genetic research. Second, the UK and many other European countries have recently moved away from culture-based diagnosis for C. trachomatis, potentially threatening the major source of purified DNA for genomic research. To alleviate this potential problem, we have been successful in developing an approach to sequence directly from clinical samples without the need for culture. In the absence of live isolates and with no routine test-of-cure this is essential if we are to have a possibility of detecting drug resistance if or when it emerges.
In the UK we have been through a period in which chlamydiologist numbers have dwindled; however, there are signs that this is changing. Considering the recent scientific gains and the threat to public health posed by this bacterium, this bodes well.
NICHOLAS R. THOMSON
Senior Scientist at the Wellcome Trust Sanger Institute (UK) in the Pathogen Genomics Group; Email [email protected]
Further reading
For more detail about the reference genomes and their comparison see Thomson & Clarke (2010). For detail of recombination see Jeffrey et al. (2010). For discussion about the link between gene loss and tissue tropism see Kari et al. (2008). For our account of the chlamydial population structure see Harris et al. (2012). The paper by Unemo et al. (2010) contains all the alert references that first announced the presence of the nvCT strain and a detailed genomic analysis if the strain itself. Seth-Smith et al. (2013) details sequencing from swabs and Wang et al. (2012) describes the new frontier of transformation in Chlamydia.
Harris, S.R. & others. (2012). Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat Genet 44, 413–419.
Jeffrey, B.M. & others (2010). Genome sequencing of recent clinical Chlamydia trachomatis strains identifies loci associated with tissue tropism and regions of apparent recombination. Infect Immun 78, 2544–2553.
Kari, L. & others (2008). Pathogenic diversity among Chlamydia trachomatis ocular strains in nonhuman primates is affected by subtle genomic variations. J Infect Dis 197, 449–456.
Seth-Smith, H.M. & others (2013). Whole-genome sequences of Chlamydia trachomatis directly from clinical samples without culture. Genome Res 22 doi:10.1101/gr.150037.112.
Thomson, N.R. & Clarke, I.N. (2010). Chlamydia trachomatis: small genome, big challenges. Future Microbiol 5, 555–561.
Unemo, M. & others. (2010). The Swedish new variant of Chlamydia trachomatis: genome sequence, morphology, cell tropism and phenotypic characterization. Microbiology 156, 1394–1404.
Wang, Y. & others (2013). Genetic transformation of a clinical (genital tract), plasmid-free isolate of Chlamydia trachomatis: engineering the plasmid as a cloning vector. PLoS One 8, e59195.
Images:
Fig. 1. Coloured scanning electron micrograph of a cultured human cervix cancer cell infected by C. trachomatis. Science Photo Library
Fig. 2. ACT (Artemis Comparison Tool) comparison of the amino-acid matches between whole C. trachomatis genomes, representing ocular (Strain Har-13), urogenital (strain UW-3) and LGV strains (strains L2 and L2b; L2b was originally thought by some to be a new variant causing disease in men who have sex with men). The genome comparison shows few differences between the strains, except for a small region (white) in the middle of the genome. This is despite the clear differences in disease and site of infection between strains. Software is available free at www.sanger.ac.uk/Software/ACT
Fig. 3. Halberstaedter and von Prowazek’s historic drawings from 1907 of a normal conjunctival epithelial cell (left), an infected cell (centre) and free chlamydial particles (right). Taken from www.chlamydiae.com
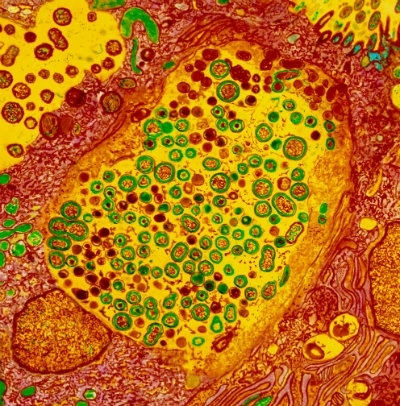
Fig. 4. Coloured transmission electron micrograph (TEM) of cells sectioned in a woman’s fallopian tube, infected with C. trachomatis. Spherical Gram-negative Chlamydia bacteria (green/brown) are seen at centre and upper left inside
cell inclusions (yellow). Dr R. Dourmashkin / Science Photo Library


