Travel and tuberculosis
08 November 2016

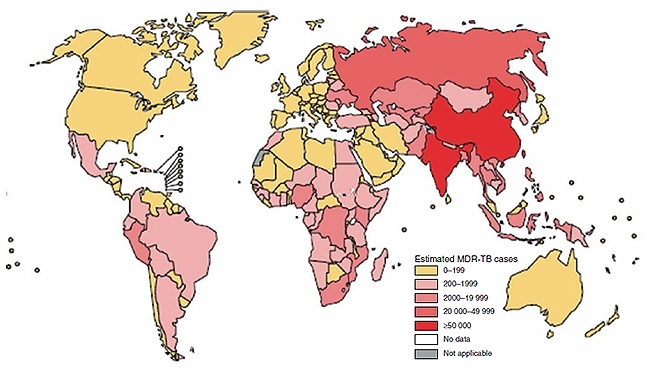
Australians frequently travel to countries with a high incidence of tuberculosis (TB). What risk does TB pose to travellers and what can be done to mitigate this risk? In the year ending June 2016, 9.7 million Australian residents left Australia visiting one or more countries for a short term period1. Of the 10 most common short term destinations, six were countries with TB incidence rates of >40 per 100 000, a threshold in common usage to define ‘high incidence’ (Table 1)2, contrasting sharply with the low TB incidence in Australia (5.3 per 100 000). Three of these destinations, China, Indonesia and India account for 45% of the world’s total number of notified TB cases. A significant burden of multi-drug resistant (MDR) TB is noted in India and China and to a lesser extent Indonesia (Figure 1). While risks of TB to travellers have recently been reviewed comprehensively elsewhere3 this short overview seeks to address key questions in understanding TB in the context of travel.
TABLE 1. TOP 10 COUNTRIES OF DESTINATION FOR SHORT TERM AUSTRALIAN TRAVELLERS (IN ORDER OF FREQUENCY)
| Country | TB incidence per 100,000 (2014) |
| New Zealand | 7.4 |
| Indonesia | 399 |
| USA | 3.1 |
| United Kingdom | 12 |
| Thailand | 171 |
| China | 68 |
| Singapore | 49 |
| Japan | 18 |
| Fiji | 67 |
| India | 167 |
FIGURE 1. NUMBER OF MDR-TB CASES ESTIMATED TO OCCUR AMONGST NOTIFIED PULMONARY TB CASES, 2014.
Assessing the risk
The likelihood of acquiring TB is determined by the probability of encountering one or more infectious cases and the cumulative duration of exposure. Host factors increase the risk that asymp- tomatic infection, (latent TB (LTB)), will progress to active disease and how severe that disease may be. In addition disease is most likely to manifest in the 2–5 years following acquisition.
The risk of acquiring infection for travellers, as assessed by tests for LTB such as the tuberculin skin test (TST) and interferon gamma release assays (IGRA), broadly is proportionate to the TB incidence in country of destination and the duration of stay. However this is not always the case and risk is substantially affected by variations in TB incidence in-country, living circumstances during the period of travel and activities pursued during travel. Participation in healthcare in countries of high burden is particu- larly acknowledged as both a risk for contracting TB and for the TB strain to be drug resistant. ‘Medical tourism’, seeking elective surgery overseas, is commonly to countries of high TB burden and may present additional risks of nosocomial or community transmission of TB.
Travellers previously treated for TB remain at risk of a new infection in communities with high rates of TB transmission.
Some of the most devastating manifestations of TB such as miliary TB and meningitis are more likely to occur in children under the age of five years and especially those less than age 2. Young children accompanying their migrant parents to countries of high TB burden to visit relatives and friends may be exposed to a significant risk of TB, especially if a household contact has untreated tuberculosis, regardless of the risk of TB as determined by overall country incidence.
Getting there and getting about
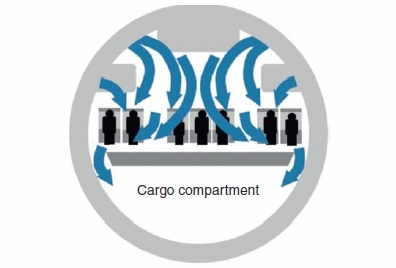
While the confined space of an aircraft may suggest an ideal environment for the spread of TB by respiratory aerosols, this is not the case. There are no published cases of active TB which have been demonstrated to have been acquired during plane travel. A recent systematic review provides evidence that infection may be transmitted on aeroplanes but 14 of 21 publications assessed did not findany evidence of transmission even when the index case was AFB smear positive4. Only one publication provided substantial evidence that TB infection (without disease) had been transmitted during air travel5. Modern passenger aircraft have sophisticated air flow management with HEPA filtered air efficiently being removed from the cabin in a downward direction (Figure 2)6. International convention considers only those passengers in the same row as an index case and 2 rows in front and two rows behind to be potentially at risk4. Contact tracing is not generally embarked upon unless the flight duration is 8 hours or longer. Compared to most countries, relatively few international flights to and from Australia and New Zealand are of shorter duration.
FIGURE 2. CABIN AIRFLOW.
While TB transmission in public ground transport may well occur, duration of exposure is short and the risk is difficult to quantify as passenger identity and detailed records of passenger seating is not usually recorded7. Crowded waiting rooms, especially if poorly ventilated could be a particular risk.
Prevention
TB prevention strategies should focus foremost on those with the greatest risk of mortality orlong term disability. As such, prevention of TB in travelling children should have the highest priority. Although no longer used routinely in Australia, BCG vaccination in early childhood reduces the risk of disseminated disease and TB meningitis by >70%. The Australian Immunisation Handbook8 recommends BCG vaccination for children, especially under the age of 5 who will be travelling and staying in countries with an annual TB incidence of 40 or greater per 100 000 population for a prolonged period. At the current time such an effective preventative strategy is not easily implemented as there is no registered BCG product available in Australia (as of 1 January 2016) and there is variable usage between State and Territory jurisdictions of ‘BCG 10’, a vaccine manufactured in Poland and not registered in Australia by the Therapeutics Goods Administration. This vaccine shortage is in the context of a global shortfall in BCG vaccine supply9, a situation which is unsatisfactory and remains unresolved.
In the absence of BCG vaccine, post travel testing for LTB is an alternative and also applicable to older children and adults where BCG is generally not recommended. Two to three months following return from a prolonged stay in one or more high TB burden countries, the TST or IGRA can be performed to assess whether LTB is present. While a pre-travel test can strengthen the conclusion that exposure has been recent, this is not necessarily required in young children from low TB burden settings such as Australia. If there is evidence of LTB, then treatment (‘chemoprophylaxis’) should be offered. For children, a three months course of isoniazid and rifampicin is well tolerated and effective. Isoniazid for 6–9 months is the most commonly used regimen in adults. The use of rifapentine-containing regimens is difficult as the agent is not registered in Australia.
Special situations
Once acquired, the risk of TB disease in HIV infected persons is generally estimated as 10% per annum as opposed to 5–10% lifetime risk for immunocompetent persons. While this risk is mitigated by immune maintenance or restoration by highly active antiretroviral therapy, HIV infected travellers should be counselled about their risk. Suggestions to use isoniazid as a pre-exposure prophylaxis for short-term travellers10 are not supported by evidence and may unnecessarily cause harm by hepatotoxicity, particularly in older subjects.
The risk of progression from latent to active TB is increased in pregnancy and is associated with a risk of congenital TB, increased risk of foetal loss as well as harm to maternal well-being11. While drug susceptible TB can be treated in pregnancy with standard therapy, some of the drugs used to treat MDR-TB are considered to be potentially teratogenic and pregnant women should consider changing their travel plans if their destination involves a prolonged stay in a community where the risk of MDR TB is high. If this is not possible, avoidance of congregate settings and non-essential visits to healthcare facilities would be prudent.
Travellers working in healthcare overseas where TB risk is increased should be educated regarding personal protective equipment use and have a baseline TST. While there is no unequivocal evidence that BCG administration to adults prevents TB, BCG can be considered for TST negative healthcare workers who are likely to work in a setting of high MDR/XDR TB burden12. For immuno-compromised people including those living with HIV and in pregnancy, BCG is contraindicated.
As IGRA tests, unlike the TST, are unaffected by prior BCG, they can be used to assess LTB in healthcare workers who are TST positive at baseline. TST negative HCWs who do not receive BCG can be monitored by TST periodically during deployment or after return. Serial IGRA testing in HCWs who are initially IGRA negative is complicated by spontaneous conversions and reversions and some authorities advise against its use in this setting13.
Post travel assessment
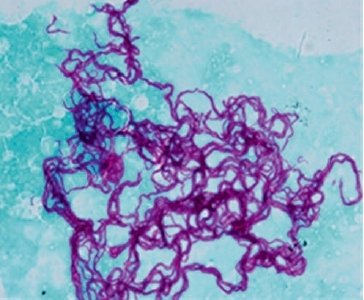
It is important that active TB is considered diagnostically at the first point of contact with the health system if a returned traveller who has visited a high TB burden country presents with TB symptoms, especially >2 weeks of cough, fevers and weight loss in adults and fevers, cough, lymphadenopathy, failure to thrive or neurological disturbance in children. Chest radiography and sputum AFB smear and culture for mycobacteria (Figure 3) and, where drug resistance is suspected, rapid molecular tests such Xpert MTB/RIF are the mainstay of the diagnostic approach. Tests of LTB should not be used to diagnose or exclude active TB as both false positive and false negative results are problematic. In contrast, evidence of recent acquisition of LTB should prompt either initiation of chemoprophylaxis or regular clinical and radiological review for at least 2 years. Australia has a well co-ordinated network for TB control and expert advice on treatment and prevention of TB can be readily obtained.
FIGURE 3. TYPICAL CORDING OF M. TUBERCULOSIS ISOLATED FROM LIQUID CULTURE.
CHRISTOPHER COULTER
Queensland Mycobacterium Reference Laboratory, Pathology Queensland, Brisbane, Qld, Australia
[email protected]
REFERENCES
- Australian Bureau of Statistics (2016) Overseas arrivals and departures, Australia, June 2016 [updated 4 August 2016]. (accessed 24 August 2016).
- World Health Organization (2015) Global tuberculosis report 2015. 20th edn. ISBN 978 92 4 156505 9 / WHO/HTM/TB/2015.22.
- Denholm, J.T. and Thevarajan, I. (2016) Tuberculosis and thetraveller: evaluating and reducing risk through travel consultation. J. Travel Med. 23, 1–6. doi:10.1093/jtm/taw031
- Kotila, SM, Payne Hallstrom, L, Jansen, N, Helbling, P and Abubakar, I (2016) Systematic review on tuberculosis transmission on aircraft and update of the European Centre for Disease Prevention and Control risk assessment guidelines for tuberculosis transmitted on aircraft (RAGIDA-TB). Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 21.
- Kenyon, T.A. et al. (1996) Transmission of multidrug-resistant Mycobacterium tuberculosis during a long airplane flight. N. Engl. J. Med. 334, 933–938. doi:10.1056/NEJM199604113341501
- European Centre for Disease Prevention and Control (2009) Risk assessment guidelines for infectious diseases transmitted on aircraft. Stockholm.
- Mohr, O. et al. (2015) Tuberculosis in public ground transport - is there enough evidence to justify contact tracing? Expert Rev. Anti Infect. Ther. 13, 1–3. doi:10.1586/14787210.2015.985656
- ATAGI (2015) The Australian immunisation handbook. 10th edn. Canberra.
- Marais, B.J. et al. (2016) Interrupted BCG vaccination is a major threat to global child health. Lancet Respir. Med. 4, 251–253. doi:10.1016/S2213-2600(16) 00099-0
- Behrens, R.H. (2016) Isoniazid prophylaxis for the prevention of travel associated tuberculosis. J. Travel Med. 23, 1. doi:10.1093/jtm/taw030
- Nguyen, H.T. et al. (2014) Tuberculosis care for pregnant women: a systematic review. BMC Infect. Dis. 14, 617. doi:10.1186/s12879-014-0617-x
- Seaworth, B.J. et al. (2014) Multidrug-resistant tuberculosis. Recommendations for reducing risk during travel for healthcare and humanitarian work. Ann. Am. Thorac. Soc. 11, 286–295. doi:10.1513/AnnalsATS.201309-312PS
- Zwerling, A. et al. (2013) Repeat IGRA testing in Canadian health workers: conversions or unexplained variability? PLoS One 8, e54748. doi:10.1371/ journal.pone.0054748


