Breaking barriers with garlic
Issue: Natural Products and Drug Discovery
05 November 2019 article

Almost every day there is another news headline calling for new, effective and affordable treatments for many of the diseases that are prevalent in society. Non-communicable diseases such as ischaemic heart disease, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), respiratory cancers and diabetes have been named as diseases needing new treatments.
Infectious diseases such as lower respiratory tract infections and tuberculosis are also highlighted in public health literature as leading causes of death, for which new and more effective treatments are needed if the effects of global health crises, such as antimicrobial resistance, are to be circumvented.
The reported health benefits of garlic are wide-ranging and touch on many of these diseases, including cancer, cardiovascular disease and infectious disease. On first thought, this apparent alignment between a problem and a possible solution sounds like a cinch. But do these reports of garlic’s health benefits stand up to scientific scrutiny so that we can use them as part of a solid evidence base for developing new treatments? And if so, what needs to be considered to transform a lowly clove of garlic into a credible, pharmaceutical intervention that complies with the drug development standards of modern- day healthcare?
The drug development process
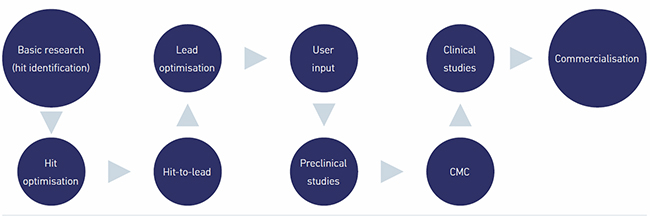
A starting point to answer these questions lies in the nature of contemporary accepted drug development processes and in an understanding of how natural products and their processing relate to these requirements (Fig. 1).
Basic research and discovery
Early drug development starts when, during hit identification stages, compounds are identified that show potential to act upon a particular symptom or disease. These hits are refined and improved during subsequent hit optimisation, hit-to-lead and lead optimisation experimentation stages, yielding compounds that can be selected to be taken into more in-depth and expensive pre-clinical studies. This in vitro work is carried out in laboratory settings and starts to shed light on if and how compounds may work.
In garlic, as in most other natural products, numerous interacting compounds are present in varying amounts across samples. This translates into a likelihood that a given compound may exhibit several interrelated mechanisms of action. For long-term scientific research, this holds much promise. In current drug development practice, it complicates the process of isolating a specific target compound for further testing and generating repeatable and acceptable data for regulators.
|
| Fig. 1. Sequence of stages in the contemporary pharmaceutical drug development process. |
While some advances have been made in understanding some of the mechanisms by which garlic exerts its influence, large gaps in our knowledge remain about how its complex and multifaceted chemistry works with other chemistry and in biological systems. This is key in being able to refine the design of subsequent experiments and strengthen one’s data package. Innovative technologies and techniques such as fluorescence imaging, high- throughput screening, microfluidics and computational chemistry advances are helping to bridge these gaps.
Although not universally done, it is always better to seek feedback from end-users of the envisioned end product. Early discussions with patients and clinicians help to avoid wasting time and money on developing a product that people will not use. With garlic, this is even more important. Garlic evokes strong emotions – people either love it or hate it – so concerns about odour or taste have to be addressed.
Pre-clinical studies
During the pre-clinical stage, data on dosing, metabolism of the compound and the compound’s toxicity is generated for use in clinical studies. Dosage is key when working with garlic, to avoid the corrosive and toxic effects that the bioactive compound allicin and its degradation product, ajoene, can have.
The question of how to get enough of the active molecule into the right place in the human body is another vital consideration. As it is originally of plant origin and a source of food for humans, garlic’s health-inducing compounds, such as allicin or ajoene, are easily and quickly broken up by the human body. This means that limited amounts of the compounds remain for long enough to be able to be taken up by the body and harnessed for medicinal purposes. This introduces a conundrum of balancing volume, concentration of bioactive molecules and the best delivery mechanism to achieve the best effect, without causing adverse effects. This is as much an art as it is a science to develop a pharmacologically active dose.
Chemistry, manufacture and controls
Chemistry, manufacture and controls (CMC) ensure that adequate amounts of optimised and approved compound are manufactured for use in upcoming clinical studies. This requires know-how in scaling up, from lab-scale experiments into commercial production. Robust and regulated quality assurance and control processes need to be in place.
Key issues to consider during CMC include how best to formulate your compound for delivery and uptake in humans. This is particularly important when working with garlic, as its active compounds break up and degrade easily when they come into contact with mammalian tissue, limiting bioavailability of the targeted compound.
Economic factors such as cost per dose administered and ease of manufacture also come into play here. Conversion of natural product extracts into druggable compounds is often complex and costly and it may therefore not make business sense to proceed.
Clinical studies
This stage evaluates the safety and efficacy of the compound in a human body rather than in a test tube. This is key as it opens the door for further product development and commercialisation.
Medicinal regulatory authorities globally are, however, often hesitant to accept medical interventions that are based on natural products. Variable amounts and concentrations of bioactive compounds across testing and manufacturing batches make it difficult to obtain repeatable results during development. Multiple mechanisms of action also reduce the ability to explain a single compound’s mechanism of action unequivocally. In practice, this means that there are no accepted processes for guiding development of natural products that are readily available to regulators, potentially requiring developers to carry out more extensive testing and increasing the cost of a compound’s development.
For many companies, such increased cost could make the difference between success and failure to achieve drug approval with their compound.
A way forward?
So, can garlic be developed into an approved medical intervention for animal or human use? Conventional wisdom would suggest that at least at conceptual level, this should be possible, at some point in time.
We are not starting from a strong evidence base, however, with lack of detail about the compound composition of garlic extracts used, variations in rigour of experimentation processes and short duration of studies being common criticisms of many of the studies that have looked at garlic’s health effects.
From a glass-half-full perspective, perhaps the answer lies in asking a better question. Advances in microbiological, chemistry and data science technologies may offer scope to strengthen this evidence base and break new ground in our understanding of garlic’s chemistry and biology interactions and potential for druggability. Perhaps the question needs to be whether developing garlic as a medicine can be part of a broader innovation in a science–business–healthcare triad. Now that would be a high-impact breakthrough.
Further reading
Block, E. Garlic and Other Alliums. The Lore and the Science. Cambridge: Royal Society of Chemistry; 2010.

Heather Graz
Chief Operating Officer, Biophys Ltd
Heather Graz has an MBA from Exeter University and before that almost 25 years of experience in direct patient care and business management. She transitioned into the biotechnology sector and has been responsible for managing commercial aspects of plant-based drug development for the past five years.
What advice would you give to somebody starting out in the field of pharmaceutical drug development?
Pharmaceutical drug development is a high risk but potentially high-impact field. It is complex and fascinating, and we are all only one cog in a very large machine. Be willing to start at the bottom, keep an open mind and learn as much as you can from the experienced people around you. Above all, remember to keep the patient, who will benefit from a successful treatment, at the forefront of what you do. They are the ultimate reason that we do what we do.
What is your greatest achievement to date?
Learning a completely new business skill set as a mature student and merging this with 25 years of prior direct patient treatment experience in a career transition from frontline speech and language therapy to managing commercial aspects of novel drug development in the biotechnology industry.
Images: Molecular model of allicin. topthailand/iStock.
Fig. 1. Heather Graz.
