Cryptococcus remains a deadly threat for those with HIV/AIDS
Issue: HIV and AIDS
06 November 2018 article
Cryptococcosis is an opportunistic fungal disease that causes life-threatening meningitis, particularly in the immunocompromised. Over the last three decades, the prevalence of cryptococcosis has increased because of the wide use of immunosuppressive drugs, the increasing number of organ transplant recipients and the HIV/AIDS epidemic.
While the majority of cases occur in sub-Saharan Africa, it remains a threat to immunosuppressed patients worldwide. Among all HIV-related deaths, 15% are caused by cryptococcal meningitis. This year, the WHO estimates that 223,100 cases of cryptococcal meningitis occurred in HIV-positive patients, with 181,000 deaths, or 81% mortality. Overall, cryptococcosis has unacceptably high morbidity and mortality rates. However, like other fungal diseases, it is underestimated and neglected despite having an alarming impact on global human health.
Cryptococcosis is attributed to several members of the genus Cryptococcus: the closely related sister species Cryptococcus neoformans and Cryptococcus deneoformans, and the more distantly related Cryptococcus gattii species complex, which mostly causes infections in hosts with fully functioning immune systems. C. neoformans is the most common cause among immunocompromised patients, even in areas where C. gattii predominates. Cryptococcal infections present as a complex syndrome that can be challenging to diagnose. Symptoms include fever, fatigue, dry cough, headache, blurred vision and confusion. Diagnosis must be confirmed by laboratory evaluation, and early diagnosis is important for positive outcomes for patients.
Challenges to diagnosis and treatment
Cryptococcal infections start with inhaled spores or desiccated yeast cells, which grow in the lungs, causing cryptococcal pneumonia. This presentation can be difficult to distinguish from other lung diseases, and can therefore go unappreciated or unrecognised in the clinic. Cryptococcal pneumonia can be fatal, and there are increasing reports of cryptococcal pneumonia in HIV-negative patients. However, in most patients, pneumonia progresses to dissemination of the fungus into the central nervous system (CNS), resulting in cryptococcal meningitis. Treatment requires combination therapy with the nephrotoxic antifungal amphotericin B (two weeks) and either flucytosine or fluconazole, followed by long-term monotherapy, representing a further continuing burden on patients and the healthcare system.
In the past, cryptococcal meningitis was uniformly fatal, but now, with more accurate diagnostic strategies and effective treatment, it has become a curable disease. Effective antiretroviral treatment (ART), to deal with the underlying HIV infection, combined with aggressive antifungal treatment has significantly improved survival. However, the high toxicity and challenging route of administration for anticryptococcal treatments mean that new treatment strategies are urgently needed. Recent clinical trials have demonstrated the value of shorter initial antifungal treatment regimens and alternatives such as itraconazole for monotherapy, and potential new drugs like VT-1129 and sertraline. Despite these improvements, we are still facing challenges: amphotericin is toxic and unaffordable, and Cryptococcus becomes rapidly resistant to the frontline antifungal fluconazole, which remains highly prescribed despite its low efficacy. These challenges demand further research into both the clinical and basic biological aspects of this human fungal pathogen.
Determinates of cryptococcal pathogenesis
C. neoformans is widespread in soil contaminated with avian excreta, particularly that of pigeons, possibly because the excreta is rich in xanthine, creatinine, urea and uric acid: nutrients that support Cryptococcus growth. C. neoformans has also been isolated from non-avian sources such as vegetable, fruit and dairy products, and the very first isolation of C. neformans was from peach juice in 1894. Cryptococcus expresses membrane-bound phenoloxidase enzymes which can convert phenolic compounds into melanin, a black pigment in the cell wall and a major pathogenicity factor. Melanin production is rare among non-pathogenic Cryptococcus species, and it has been been proven to protect cryptococcal cells from attack by host immune effectors cells.
Fig. 1. (a) India ink staining of Cryptococcus neoformans grown under Titan cells induction conditions (cells >10 μm, Titan cells, cells around 5 μm, yeast cells). (b) Murine macrophages (J774) infected with cryptococcal cells. Red, macrophages; blue, cryptococcal cells; green, capsule structure; white arrows, engulfed yeast cells; grey arrows, unengulfed Titan cells.
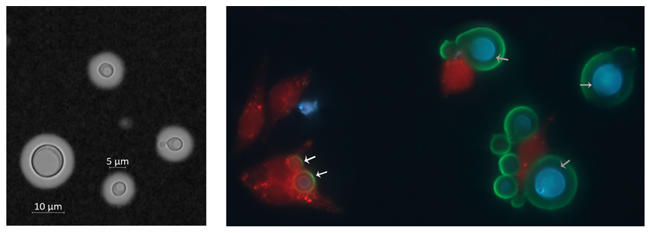
C. neoformans yeast cells are around 5 µm in diameter, with a round-to-oval shape. In patients, it reproduces by budding. In the environment, it can also form mycelia when mating, with formation of basidiospores at the end of invasive hyphae. Under certain conditions, including within patients, or growth in low glucose with 5% carbon dioxide and serum, it can produce a characteristic structure at the outer layer of the cell wall, the polysaccharide capsule (Fig. 1a). This capsule can extend from the cell, ranging from 1 to 30 µm in diameter, and is a defining feature of the disease.
Many diagnostic tests are specifically designed to identify the presence of the capsule. Classic identification has been through India ink staining (pictured), in which ink reveals the polysaccharide capsule around cryptococcal cells. This method allows the rapid and easy identification of cryptocococcal cells from cerebrospinal fluid, although it lacks sensitivity at the early infection stage and may miss around 20% of patients with culture-positive cryptococcal meningitis. More recently, the CrAg latex agglutination and lateral flow assays detect the presence of capsule antigen in the blood. In terms of the sensitivity of diagnosis, these tests offer specificity close to 100%, although cases can occasionally still be missed. Unlike the conventional culture and microscopy methods, CrAg-dependent tests require only minimally specialised laboratory facilities and low costs.
Titan cells influence disease progression
Cryptococcosis starts when small cells – like desiccated, encapsulated yeast cells or basidiospores – are inhaled. In order to penetrate deeply into the lung parenchyma, cells must be smaller than 2 µm, while normally yeast cells are around 5 µm in diameter. It is therefore postulated that basidiospores are the infectious propagule, due to their small size (1.8–2 µm in diameter) and inherent stress resistance. C. neoformans has several intrinsic features, such as a melanin coat and capsule, that enable survival within the host environment. Inhaled cells will also encounter host immune cells including macrophages, neutrophils and dendritic cells among others, which all can kill the fungus directly or indirectly. However, cryptococcal cells can escape from host defenses via several mechanisms, including hijacking host phagocytes or evading uptake through an unusual change in cell size: the switch from yeast to Titan cells. This yeast-to-Titan transition happens during the earliest infection stage. During this transition, cryptococcal cells dramatically increase in size and repeatedly replicate their DNA, ending with formation of very large (more than 10 µm, up to 100 µm diameter) and highly polyploid Titan cells (Fig. 1b). These Titan cells were recognised as a specific in vivo phenomenon only in the last decade, but their importance in disease is becoming increasingly clear. Titan cells are thought to mediate pathogenicity through several distinguishing features: an altered cell wall structure, highly compacted capsule and viability under high antimicrobial stress. Very recently, in vitro models for Titan cells have been reported, and this innovation opens up important new avenues of study for pathogenicity and drug development.
As a novel virulence factor, the importance of Titan cell production in disease progression has become well recognised. Titan cell formation can help C. neoformans establish infection in lungs rather than being cleared by host immune cells, as Titan cells are more resistant to phagocytosis (Fig. 1b). Meanwhile, these enlarged cells also protect small cells from being engulfed and killed, resulting in an overall increase in fungal burden. These small cells are also more drug resistant, and this together with their small size is thought to help them further disseminate into the CNS. In addition, the production of Titan cells activates a non-protective type 2 helper T (TH2) cell response in the host, further contributing to fungal pathogenesis.
Conclusion
Cryptococcosis is a serious, widespread fungal disease that causes unacceptably high mortality in HIV patients every year. Refinements in diagnosis and treatment raise the possibility of improving outcomes for patients, but significant challenges remain. We urge further research into the basic biology of this pathogen, including improved understanding of the role of the yeast-to-Titan switch in disease progression and drug resistance.
Further reading
Bicanic, T. & others (2006). Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis 43, 1069–1073. doi:10.1086/507895
Cryptococcal disease: what’s new and important. World Health Organization. www.who.int/hiv/mediacentre/news/cryptococcal-disease-key-messages. Last accessed 5 September 2018.
Molloy, S.F. & others (2018). Antifungal combinations for treatment of cryptococcal meningitis in Africa. New Eng J Med 378 1004–1017. doi:10.1056/NEJMoa1710922
Zhou, X. & Ballou, E.R. (2018). The Cryptococcus neoformans Titan cell: from in vivo phenomenon to in vitro model. Curr Clin Microbiol Rep 1–9. doi:10.1007/ s40588-018-0107-9

Xin Zhou
Institute of Microbiology and Infection, School of Biosciences, University of Birmingham, Birmingham, UK
[email protected]
Twitter: @Phoebezhou07
Xin Zhou is a first year PhD student working in Dr Elizabeth Ballou’s lab. Her research focuses on the mechanisms of Titansation in Cryptococcus neoformans and its effects on host and pathogen interactions.

Robin C. May
Institute of Microbiology and Infection, School of Biosciences, University of Birmingham, Birmingham, UK
Robin C. May is Professor of Infectious Diseases and Director of the Institute of Microbiology & Infection at the University of Birmingham, UK. His research interests focus on host-pathogen interactions and, in particular, in understanding how some fungal pathogens are able to subvert the innate immune system.

Elizabeth R. Ballou
Institute of Microbiology and Infection, School of Biosciences, University of Birmingham, Birmingham, UK
[email protected]
Twitter: @BallouLab
Elizabeth R. Ballou is a Lecturer in Cellular Microbiology and Wellcome Trust Sir Henry Dale Fellow at the University of Birmingham. She studies how fungi respond to environmental signals to cause disease, focusing on the molecular mechanisms of the yeast-to-Titan transition by Cryptococcus neoformans.
What does a typical day (or week) involve for you?
Robin: I really enjoy the fact that no two days are ever the same.
Xin: As a first year PhD student, every day I start by planning my lab work, and then follow my list in the lab.
Liz: One of my favourite things about being a scientist is that there is no such thing as a typical day.
What inspired you to become a microbiologist?
Robin: I blame children’s television! I have a memory of watching a TV programme which had a special episode on ‘germs’. I was so captivated that I spent an hour hanging over a bucket, thinking that if I vomited I’d be able to spot some evil cartoon bug!
Xin: I first encountered different kinds of microbes as an undergraduate. I was attracted by these beautiful organisms, and later, during a lab training programme, started to realise how important microbes are for our lives.
Liz: I was lucky to grow up around microbiologists. They cemented my belief that through research we have an enormous opportunity to improve human health.
Images: Coloured scanning electron micrograph of Cryptococcus neoformans. Magnification: x1,200 when shortest axis printed at 25 millimetres. Dennis Kunkel Microscopy/Science Photo Library.
Fig 1. Xin Zhou
