Comment: Every microbe matters: making movies of single cells
Issue: Imaging
13 February 2018 article
The first time human eyes saw a microbe was through the microscopes of Antonie van Leeuwenhoek in the 1670s. His microscopes may have looked crude by modern standards, but were powerful enough to distinguish individual cells of fungi, protists and even bacteria. Van Leeuwenhoek’s contemporaries were astounded by the existence of this world of tiny creatures with strange forms and going about on mysterious errands.
In the following centuries, microscopes were used to compile a basic natural history of microbes. They could not be used for much more. Many of the key discoveries that gave birth to microbiology in the 19th century, and to molecular biology in the 20th century, largely bypassed microscopy. Bulk-level studies of pure cultures, in which billions of cells from a single strain are cultured and assayed together, revealed a treasure trove of intracellular biochemistry. As a side effect, we also came to think there was a sameness about how individual microbial cells behave.
In recent years, advances in microscopy, such as increased automation of imaging, fluorescent proteins, computational analysis and microfabrication, forced us to revise our view and realise that microbial cells also have their own individual distinctiveness. At the same time, this distinctiveness can often enable decisions that work for the benefit of a colony as a whole. Static images, isolated snapshots of a particular moment in the life of the colony, offer glimpses of microbial individuality. Time-lapse imaging, or movies, of single cells bring this individuality into full view, tracking it regularly through the lifetime of a microbe. Our lab and others have been using microscopy movies to understand how single cells make decisions, and what lies at the origin of these decisions. They may owe to the microbe’s internal state, or be due to extracellular circumstances, be it neighbouring cells or environmental cues.
How to be a movie-maker
The process of single-cell movie- making is straightforward, but each step presents technical challenges. First, live cells are laid in a support medium, where they continue to grow and divide, while sitting for hours or days on a microscope stage. An automated set-up snaps and stores pictures at regular intervals. Next, computer software analyses these pictures, creating maps that demarcate the boundaries of individual cells, and tracking which cell is which through consecutive movie frames. Typically, each microbe has been transformed to carry sequences of DNA coding for fluorescent proteins that glow under the appropriate wavelength of light. Finally, by superimposing this glow onto the cell maps, we can measure how much protein, or gene expression, there is in each cell over time.
What single-cell movies can tell us
Why bother measuring single-cell gene expression through time? The main reason is that by finding out what individual cells are doing, we can discover cell behaviours that are hidden in population averages. Recently, our lab used single-cell movies to reveal how certain genes in photosynthetic bacteria are turned on twice every day. These genes are controlled by an internal 24-hour clock, which completes only one full cycle every day, as our own body clock does. It was therefore surprising that certain genes can complete two cycles per day, but, because each cell is not in perfect synchrony with its neighbours, the collective average masks this behaviour away. Ignoring the individuality of each cell causes us to see only one cycle, when in fact there are two.
Single-cell movies of fluorescent proteins can therefore reveal large variability from one microbe cell to the next, even for genetically identical cells in the same environment. At first, it was thought that this variability, or noise, was a nuisance caused by low protein numbers and the small cellular volume of microbes. However, it has become clear that this cell-to-cell noise can provide a function. We are only just beginning to understand the range of ways through which individual cell dynamics can shape population-level behaviours.
Single-cell individuality and collective function
Natural microenvironments change in unpredictable ways, and it may be impossible for any one microbe to respond (by activating the appropriate genetic programme) in time. For example, bacteria are often attacked with antibiotics. If the dose is high enough, all normal cells die. Yet, after the antibiotic is washed away, the population occasionally returns, in the clinic or in the lab. Time-lapse microscopy lays bare the strategy bacteria use to cope and survive. Just like any person taking an insurance, weighing a financial commitment in the present against the risk of catastrophic failure in the future, the bacterial colony survives by setting aside a small number of cells that enter a state called persistence, a kind of dormancy. These individual bacteria cannot grow, and therefore reduce colony yield, but at the same time, dormant cells can cope with antibiotic stress. Single-cell movies that have, by now, become classics show rare cells randomly entering, and later exiting, that dormancy state. When an antibiotic stress comes in, all other cells die, but persisters later emerge from dormancy, behaving like normal cells and quickly reproduce to regenerate the colony.
Greater control with microfluidics
Single-cell imaging is not just about microscopes and cells. One of the allures of the technique is the possibility of watching in real-time how individual microbes react to quick changes in their environment, like in the case of persister cells. To accomplish this, the skills of physicists and engineers have made way into the microbiology lab. One sign of this ‘invasion’ is the rise of experimentation in microfluidics. Microfluidic chips are small devices, made with a type of silicone polymer, of micrometre-size channels that have been designed to fit cells in a single layer. A liquid broth with nutrients or stress-inducing chemicals is flown continuously through the micro-colony, and can be swapped instantly with a broth with a different composition at pre-specified times. Many groups, including our own, employ a contraption that lets most cells be washed out with the flow, but retain some others in place. These cells can then be imaged for, in principle, an unlimited time. Rare events, or processes that develop over a long time, such as how single cells age, can be studied in unprecedented detail. Other groups are using special devices with wider chambers to study the treks of single cells, allowing us to understand how bacteria move away from threats or towards targets.
New frontiers
A major goal in the field of biology is, of course, to understand how cells function in real natural environments. In nature, many of our favourite microbes live in densely packed communities called biofilms, where they engage in behaviours not seen elsewhere and where cell-to-cell diversity is high. However, biofilms present a considerable challenge to single-cell time-lapse imaging. Cells can become aligned in any direction in 3D space, making their boundaries hard to map. Traditional wide-field microscopy illuminates the sample evenly and only scratches the surface of the biofilm. New computational techniques applied to confocal microscopy, which blocks out-of-focus light and can see through masses of cells, have just begun to penetrate into this secluded world of microbial individuality. As we keep pushing these and other boundaries, single-cell movies will continue to reveal the complex and dynamical lives of microbes.
Further reading
Balaban, N. Q. & others. (2004). Bacterial persistence as a phenotypic switch. Science 305, 1622–1625.
Grünberger, A., Wiechert, W. & Kohlheyer, D. (2014). Single-cell microfluidics: opportunity for bioprocess development. Curr Opin Biotechnol 29, 15–23.
Locke, J. C. W. & Elowitz, M. B. (2009). Using movies to analyse gene circuit dynamics in single cells. Nat Rev Microbiol 7, 383–392.
Martins, B. M. & others (2016). Frequency doubling in the cyanobacterial circadian clock. Mol Syst Biol 12, 896.
Yan, J. & others (2016). Vibrio cholerae biofilm growth program and architecture revealed by single-cell live imaging. Proc Natl Acad Sci U S A 113, E5337–E5343.
Bruno Martins
Sainsbury Laboratory, University of Cambridge, Cambridge CB2 1LR
Bruno Martins was originally trained in Physics, before embarking on a PhD in Systems Biology at the University of Edinburgh. Later, he moved to the University of Cambridge, where he has been studying the cyanobacterial circadian clock using quantitative single-cell approaches, as well as synthetic biology.
James Locke
Sainsbury Laboratory, University of Cambridge, Cambridge CB2 1LR
James Locke is a group leader at the Sainsbury Laboratory at the University of Cambridge. His group is using movies to examine gene expression at the single-cell level in bacteria, cyanobacteria and plants.
What do you love most about your job?
James: Moving to the Sainsbury lab, where everyone tries to collaborate rather than compete, has been a revelation.
Could you describe a typical workday?
Bruno: On an ideal day, I organise my time around starting a microscopy movie. The microscope room remains a magical place for me. Every time I bring a sample into focus, I feel a rush of excitement in anticipation of what I might see. Time-lapse imaging is fully automated these days, so my attention is quickly drawn to making sure the computer scripts we wrote to control the microscope are correct. Once the experiment is running, I sit at my desk and analyse last week's experiment. Computer software processes the images and extracts the raw data. The real fun starts later, when I get to apply different mathematical tricks in search of patterns that may not be obvious at first sight. Our lab combines experiments with theory, and I have a background in physics myself. So, on a lucky day, the data will be intriguing enough to warrant finding a quiet room to start sketching some equations for a mathematical model. I simulate it on a computer the next day.
On an actual typical day, setting the time-lapse movie was easy enough, but I noticed a problem with last week's experiment, so its data is useless. Or maybe its results were inconclusive. Either way, it's back to the drawing board. Frustrated, I pick a paper from the backlog pile on my desk, go grab a coffee, and try again tomorrow.
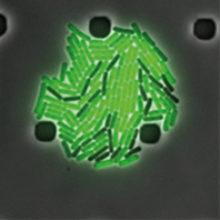
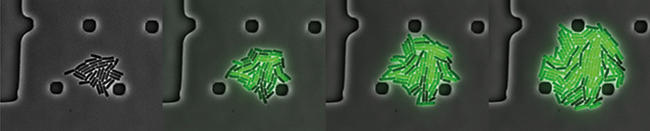
Image: Time-lapse of a B. subtilis colony in a microfluidics chamber shows different individual strategies in response to a chemical stress (green cells turned a gene reported on, dark cells kept the same gene reporter off). Darker shades in the background are structural features of the chamber. Christian Schwall, University of Cambridge.
