How do you image an exotic virus?
Issue: Imaging
13 February 2018 article
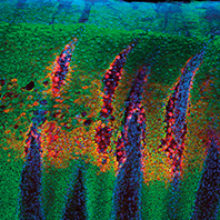
The internationally unique bioimaging facility at The Pirbright Institute enables us to explore the biology of animal diseases, and see how some of the world’s most devastating livestock viruses function on a microscopic scale.
Disease outbreaks in livestock have a major impact on economies, health and food security globally every year. This is particularly true in poorer areas where diseases are endemic and communities rely on livestock for survival. With little money available for research and few vaccines available for prevention, eradication of disease is challenging.
Countries that are disease free are still susceptible to virus incursions, the cost of which can escalate rapidly when knock-on effects such as restrictions on trade and movement are taken into account. For example, the 2001 foot-and-mouth disease (FMD) outbreak in the UK is estimated to have cost over £8 billion.
The threat of virus outbreaks is always present and can occur from illegal movement of animals, interaction of domestic herds with wild animals or spread by vectors via insect or tick bites. Border control authorities keep a close eye on imports; however, wildlife and vector species know no borders. This has been brought into sharp relief in recent years with the importation of African swine fever virus into the Republic of Georgia in 2007 and its subsequent spread across the Russian Federation, Eastern Europe and into the European Union. Taking into account that this disease causes up to 100% mortality rate in domestic pigs and there is currently no effective vaccine, other countries including China, home to half the world’s pig population, are preparing for the worst.
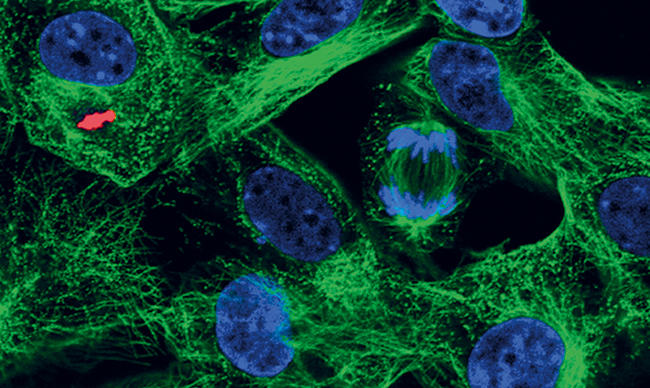
The North Sea provides a corridor of protection for the UK, although this is not infallible. In 2007, the bluetongue virus outbreak across continental Europe reached UK shores as clouds of small biting midges carrying the virus were blown over the Channel, arriving in Suffolk in August. A successful sheep and cattle vaccination programme eradicated this virus from the UK within a year.
Given the devastating effects an outbreak can have at a national and personal level, it is imperative that effective vaccines are developed and used to either stop an outbreak in its tracks or, better still, prevent it from happening in the first place. The work here at The Pirbright Institute is focused on research into new vaccines, diagnosis, and reporting of global outbreaks and prediction of future threats to the UK.
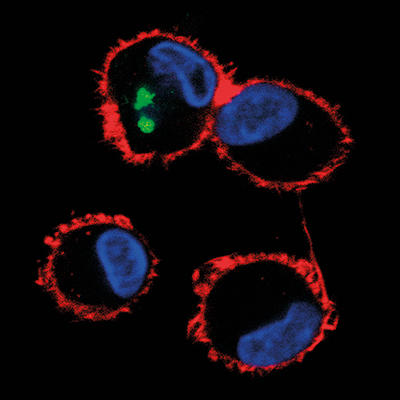
Why use microscopy to study viruses?
In order to produce an effective anti-viral treatment, the basics of how a virus infects a host animal, how it replicates and how it spreads must be identified. This is where microscopy comes into its own. A combination of high- resolution light microscopy and electron microscopy can give protein localisation and ultrastructural information that other molecular laboratory techniques cannot. The majority of projects using microscopy at the Institute seek to answer the age-old question of how does a virus infect a cell and take over its machinery to reproduce? If those pathways are defined in detail then the possibility of blocking one of the processes, and therefore infection, can be investigated and the data ultimately used to produce a vaccine.
Sounds easy, doesn’t it?
As with all research, there are challenges associated with delivering results. However, there is an added layer of complexity at The Pirbright Institute because our confocal and electron microscopes are located within a high containment facility. The Biotechnology and Biological Sciences Research Council National Virology Centre: The Plowright Building accommodates laboratories within a restricted access area where we can research live veterinary viruses that are exotic to the UK. These labs are designed for the use of SAPO4 (Specified Animal Pathogen Order level 4) viruses, the most well-known and economically important of which being foot-and-mouth disease virus (FMDV). In practical terms, anything that goes into these labs cannot leave without being fully decontaminated. Air is passed through double HEPA filters before leaving the building, effluent is treated on site, waste is autoclaved or fumigated out, and staff have to take a full shower before leaving. The labs are kept under negative pressure so that air is sucked in, never blown out. This leads to some interesting practices, not least of which is that the first thing you do after arriving at work is take your clothes off! Luckily, the second thing you do is put on some blue scrubs before accessing the laboratories.
The Bioimaging Suite in the restricted area houses confocal and super-resolution microscopes, and two transmission electron microscopes (TEM), plus there are areas for tissue culture, sample preparation and image analysis. The challenges of running highly complex equipment within a containment building are many, and it takes years of experience to understand and apply the biosafety regulations to all that we do. Top of the list of considerations is decontamination. How do we effectively decontaminate complex equipment to comply with the stringent terms of our SAPO licence? To address this, all possible situations are risk assessed and protocols are written so that users know what to do, should the situation arise. For example, we have procedures in place to follow if:
- A microscope needs cleaning.
- A microscope needs maintaining by a contract engineer.
- There is a small virus spill on the confocal microscope stage or inside the TEM column.
- A part needs to be sent away for repair.
- Parts are broken and need to be disposed of.
Procedures include wiping with disinfectant, fumigation with formaldehyde gas (without the use of ammonia as a neutralising agent) and gassing with chlorine dioxide in the case of the TEM vacuum system. It is rare that parts are decontaminated and sent out for repair. If that does happen, the serial number of each particular part is recorded so that we can make sure the same part is returned. Parts will more usually be replaced by manufacturers, which of course adds a premium to the cost of our instrument service contracts.
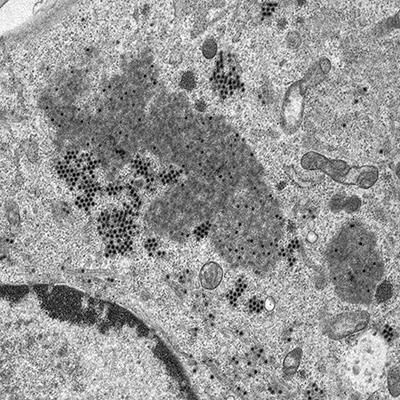
Many preparation techniques for both confocal and electron microscopy involve the use of aldehyde fixatives, which deactivate viruses. These samples can be imaged with no further biosafety concerns. However, certain imaging methods require live samples, for example live cell imaging in the confocal microscope. Each method is risk-assessed, and procedures are put in place which minimise or eliminate the risk of live virus becoming uncontrolled.
It is generally accepted that preparation techniques for the electron microscope that eliminate the use of fixatives provide more accurate information about your sample. These modern cryo techniques are essential in any EM lab, but lead to biosafety complications. One technique calls for live, infected samples to be frozen at a pressure of 2,100 bar in order to preserve the fine ultrastructure, and cryoTEM techniques dictate that live virus samples should be plunged into liquid nitrogen-cooled ethane and imaged at a very low temperature in the TEM. In order for us to carry out these essential preparation techniques safely, and in compliance with our licence, each has to be thoroughly risk assessed and additional steps incorporated to eliminate the potential for uncontrolled virus.
Is it worth it… really?
Of course! Being able to contribute to improving the health and well-being of livestock, and the people who rely on livestock, on a global scale is hugely rewarding. Vaccines that are developed by scientists at The Pirbright Institute have a proven impact. For example, in 2010 the UN Food and Agriculture Organization (FAO) confirmed the global eradication, through the use of vaccines developed at The Pirbright Institute, of rinderpest, a disease that had devastated domestic cattle populations in Europe, Africa and Asia for centuries.
The day-to-day challenges of running a multi-user advanced microscopy facility in a high containment building are numerous, but they are not insurmountable given access to the right infrastructure and expertise. After all, if it were easy, everyone would be doing it!
Now if you’ll excuse me, I’m off for a shower…

Pippa Hawes
Head of Bioimaging, The Pirbright Institute, Ash Road, Pirbright, Surrey GU24 0NF
[email protected]
Facebook: ThePirbrightInstitute
Twitter: @Pirbright_Inst
An experienced, qualified microscopist with an established track-record in imaging virally-infected animal cells and tissue, Pippa Hawes has specific expertise in confocal and electron microscopy, and its application to the study of virus–host interactions. Pippa is regularly invited to speak at residential courses and events in the UK and further afield. Well known within the microscopy community, she is a member of the Council of the Royal Microscopical Society and an active supporter of microscopy networks, such as EM-UK and BioimagingUK.
What inspired you to become a microbiologist?
My career path has been slightly different from that of a classic scientific researcher. During my first degree at Royal Holloway, University of London (BSc Zoology), I was lucky enough to use their electron microscopes. I enjoyed the intricacies of specimen preparation and the challenge of coaxing the best possible images out of the microscopes, but mostly I became fascinated with looking at cells, the building blocks of all living things. After my degree, the opportunity arose for me to do a MSc in Biological Electron Microscopy at Aberystwyth University, which defined my career. I began working at The Pirbright Institute in 2001 as a microscopist, with very little experience in microbiology. Since then, I have completed my PhD, and learnt a lot about viruses along the way – but I wouldn’t call myself a virologist!
What is the most rewarding part of your job?
I still enjoy using our microscopes to investigate how viruses interact with host cells, but now I get more satisfaction from discussions with our scientists about projects that include microscopy, and the questions that can be answered. We have a unique facility here, so there are almost endless opportunities to add value to projects. If I had my way, everyone would have a microscopy element in their research!
Images: The article's thumbnail is a bovine tongue epithelium, infected with foot-and-mouth disease virus, imaged using a confocal laser scanning microscope. The second image is a confocal laser scanning microscope image of cells in culture infected with African swine fever virus, labelled with an antibody against a viral protein (red), an antibody against microtubules (green) and stained for DNA (blue). A dividing cell can be seen in the top right quadrant of the image. The third, alveolar macrophages infected with African swine fever virus, labelled with an antibody against a viral protein (green) and stained for the actin cytoskeleton (red). The cell nuclei, containing DNA, are coloured blue. This image was taken using a confocal laser scanning microscope. And lastly, a cell infected with bluetongue virus, and then prepared for the transmission electron microscope. New progeny virus appears as small, black dots in the cell cytoplasm. Part of the cell nucleus can be seen in the lower left-hand quadrant of the image. All images are from the Pirbright Institute.
