The curious meeting of two partners: the squid–vibrio symbiosis
Issue: Light
11 August 2015 article

2012 marked the centennial anniversary of Stillman Berry’s description of a species of Hawaiian bobtail squid named Euprymna scolopes. The original squid species samples, collected during an expedition sponsored by the United States Bureau of Fisheries, were obtained near the island of Molokai in the Hawaiian archipelago. A later report highlighted that the squid are quite prevalent among all the Hawaiian Islands. In fact, these nocturnal animals can even be found swimming in shallow water within inches of the shoreline in contrast to other similar species that are usually found at much greater depths.
Berry could not have imagined at the time of his reports that this small squid would facilitate our understanding of how symbioses between animals and bacteria develop and evolve. Euprymna scolopes, like many other nocturnal marine animals, produces light for a behaviour called ‘counter-illumination’. By illuminating the seafloor with light emitted from the ventral side of its mantle, E. scolopes can disrupt the shadow cast by moonlight or starlight and consequently avoid detection from below. This light, also known as bioluminescence, is emitted by populations of a marine bacterium called Vibrio fischeri that E. scolopes houses within a dedicated structure called the ‘light organ’. Research of this mutualistic association over the past 25 years has revealed insight into the core principles associated with co-evolved host–microbe interactions.
Squid husbandry and V. fischeri genetics
Key to the success of the squid–vibrio symbiosis field has been the ability to study the two partners independently. V. fischeri transmission is horizontal, i.e. E. scolopes juvenile squid hatch from their eggs un-colonised and acquire V. fischeri symbionts from the surrounding seawater. Approximately every 2 weeks, an E. scolopes female lays a clutch of eggs that can number in the hundreds. Within 3–4 weeks, the mature embryos will hatch and can be raised ‘apo-symbiotically’, or in the absence of V. fischeri. Such apo-symbiotic samples have enabled researchers to control for the changes in the host that are independent of V. fischeri colonisation.
V. fischeri is a gammaproteobacterium, and many of the molecular tools generated for use in Escherichia coli have been directly implemented in studying V. fischeri. In particular, transposon mutagenesis, allelic exchange vectors, and gene expression reporters have been instrumental in parsing the bacterial factors involved in symbiosis. Within the last decade, the genome sequencing of various V. fischeri strains has also provided insight into co-evolution of mutualism. For instance, in 2009, Dr Mark Mandel (Northwestern University) and colleagues at the University of Wisconsin-Madison discovered that the acquisition of a gene encoding a regulatory protein by a non-symbiotic strain of V. fischeri enabled the bacterium to colonise the squid light organ.
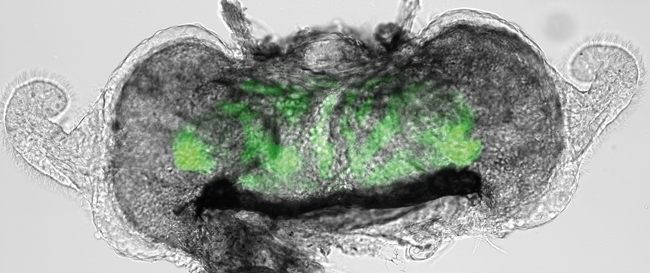
The light organ and the ‘exclusive contract’
The light organ represents an exquisite result of co-evolution between an animal and its microbial symbiont. The light organ is a bi-lobed structure that appears during embryogenesis. Within hours of hatching, the squid acquires V. fischeri symbionts from the surrounding seawater. Individual V. fischeri cells initially attach to cilia located on the surface of the light organ and are subsequently propelled to three pores located on each side. Using flagella-based motility, V. fischeri cells swim through the pores and eventually enter epithelial-lined diverticula, referred to as crypt spaces. In return for their bioluminescence, populations of V. fischeri located within these crypt spaces receive shelter and peptides from the squid host. The light organ also consists of reflector and lens tissues that guide the light away from the mantle cavity.
A pivotal moment that launched the squid–vibrio symbiosis into the spotlight was reported in 1991 by Drs Margaret J. McFall-Ngai and Edward (Ned) G. Ruby (University of Wisconsin-Madison), then at the University of Southern California. They had discovered that colonisation of the nascent light organ by V. fischeri resulted in massive alterations in the organ, including loss of ciliated, microvillous surfaces associated with the arm-like appendages protruding from the organ. Furthermore, the host epithelial cells comprising the appendages undergo apoptosis upon bacterial colonisation and have completely regressed within adult animals. A subsequent study showed that lipopolysaccharide and peptidoglycan components shed by V. fischeri are responsible for this regression. The monomer of peptidoglycan is also known as tracheal cytotoxin and involved in the pathogenesis of Bordetella pertussis and Neisseria gonorrhoeae. These compelling examples of how beneficial microbes, and their conserved microbial components, can shape the developmental programme of animals have had a resounding impact on current research, including the gut microbiota research field.
Quorum sensing and light production
Gene expression has been a focus of many laboratories studying the squid–vibrio symbiosis. Within the light organ, V. fischeri cells coordinate light production using quorum sensing, which describes an intracellular form of communication that involves the synthesis, export and detection of small signalling molecules, called autoinducers. The primary receptor of the autoinducer is a transcription factor, LuxR, which activates transcription of the lux genes that encode the enzyme responsible for bioluminescence. In essence, V. fischeri can use the autoinducer concentration as a measure of cell density, thereby only producing light when there is a sufficient number of cells. The cells are so sensitive to autoinducers that researchers often refer to these signalling molecules as bacterial pheromones.
Bioluminescence appears to be the primary function of V. fischeri while in symbiosis with E. scolopes. Mutants of V. fischeri that are deficient in light production, e.g. mutants lack either the lux genes or ability to respond to quorum sensing, are rejected by the host. More recently, evidence that the squid host prefers dim strains of V. fischeri has emerged. For instance, Dr Cheryl Whistler (University of New Hampshire) and colleagues found that introduction of bright V. fischeri strains that propagate within the host eventually yield dim variants of V. fischeri. Future experiments to characterise the mutations associated with these dim phenotypes may provide further knowledge into the selective pressures experienced by V. fischeri inside of the light organ.
Scientific education
In some ways, the topic of host–microbe interactions represents the culmination of an undergraduate degree in life sciences. Courses in microbiology, immunology, pathogenesis, evolution, biochemistry and molecular biology typically contain content associated with host–microbe interactions. Due to the binary nature of the partner association, the squid–vibrio symbiosis has emerged as a useful and exciting system to model non-pathogenic host–microbe interactions in the classroom. Rather than causing a disease, infection of E. scolopes by V. fischeri provides the benefit of light production to the host. Various bacterial phenotypes, e.g. motility and bioluminescence, are easily explored using V. fischeri within the classroom and laboratory.
Future directions
The squid–vibrio symbiosis continues to offer researchers the opportunity to explore the basic principles underlying highly specific host–microbe interactions. Sequencing the genomes of E. scolopes and other Euprymna spp. will allow the use of comparative genomics approaches to reveal novel host-derived factors that promote symbiont specificity. Single-cell imaging and transcriptomics approaches promise new insight into the population dynamics that occur among V. fischeri cells. Together, these approaches that use the squid–vibrio symbiosis will continue to reveal the mechanisms underlying animal–microbial symbioses.
TIM MIYASHIRO
Biochemistry and Molecular Biology Department, Penn State University, 410 S. Frear Building, University Park, PA 16801, USA
[email protected]
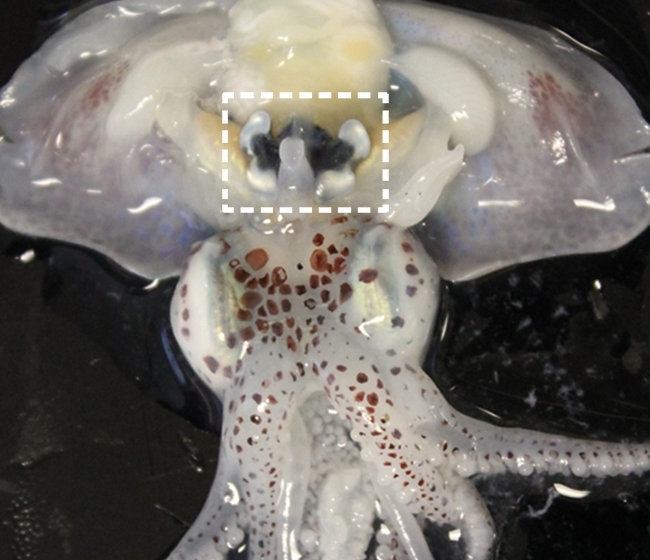
Image: Adult E. scolopes collected in offshore water in Oahu, Hawaii. Light organ extracted from a juvenile squid. Fluorescently labelled V. fischeri populations are shown in green. Anaesthetised adult E. scolopes with dissection in ventral side of mantle – light organ boxed. T. Miyashiro..
