The quest for COVID-19 treatments
Issue: SARS-CoV-2 and COVID-19
12 October 2021 article
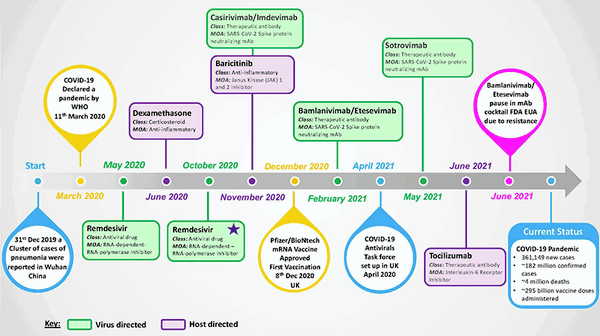
Treatments for coronavirus disease 19 (COVID-19) were not available at the outset of the pandemic caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), which emerged as a new pathogenic human respiratory virus in late 2019 and reached pandemic status by March 2020 (Fig. 1). The urgent need for COVID-19 treatments was quickly recognised, as 5–10% of patients develop a severe disease that requires hospitalisation/intensive care and around 4 million deaths have been recorded by the World Health Organization (WHO) to date.
Scientists around the world rapidly began the quest for effective COVID-19 treatments. In the first instance, efforts focused on clinical trials to test existing drugs in the hope that they could be rapidly repurposed for treatment of COVID-19 patients. Examples of these clinical trials include the UK-led RECOVERY trial and WHO-led Solidarity trial. A small number of treatments have now been deemed safe and offer some therapeutic benefits against COVID-19, and have thus been granted approval or emergency use authorisation (EUA) by regulatory bodies such as the US Food and Drug Administration (FDA). A huge number of other prospective therapeutic agents are currently undergoing investigation as potential COVID-19 treatments.
In this article, we highlight key treatment successes and failures and review different virus-targeted and host-targeted treatment strategies (Fig. 1). We discuss ongoing and future COVID-19 treatment research approaches and perspectives, as despite the ongoing successful rollout of COVID-19 vaccines, effective treatment options will continue to be vital for patients who succumb to serious disease and to tackle the clinical consequences of long-COVID.
Targeting the virus
Antiviral drugs have been successfully developed and used against a handful of clinically important viruses and are an essential approach for viral disease treatment. Typically, antiviral drugs are highly optimised to act against a specific virus by directly targeting a viral component or step in the virus replication cycle. Unfortunately, prior to the pandemic, no specific antiviral drugs had been approved for clinical use against human coronaviruses.
The quickest strategy to identify antiviral drugs to treat COVID-19 is investigation of existing compounds with known broadly acting antiviral activity against a range of RNA viruses including other coronaviruses. The leading candidate fitting these criteria was remdesivir (Veklury), which belongs to the established drug class nucleos(t)ide analogue inhibitors. Drugs in this class target the viral RNA-dependent RNA polymerase, preventing viral RNA genome replication. Laboratory/animal studies demonstrated that remdesivir exhibits anti-SARS-CoV-2 activity, but clinical trials have reported mixed results with respect to its therapeutic effect in COVID-19 patients. Despite this, remdesivir is currently the only directly acting small-molecule antiviral drug to receive FDA approval for COVID-19 treatment. To build on a potentially successful strategy, other nucleos(t)ide analogues are also under investigation, such as favipiravir (Avigan), an antiviral drug licenced in Japan to treat influenza.
The concept of repurposing existing drugs for fast discovery of COVID-19 treatments was also applied to the HIV protease inhibitor lopinavir, based on evidence from laboratory/animal studies that the drug exhibits activity against SARS-CoV and MERS-CoV. Clinically, lopinavir is administered in combination with ritonavir, to boost lopinavir’s plasma half-life. Unfortunately, numerous clinical trials demonstrated no benefit from lopinavir–ritonavir (Kaletra) treatment in COVID-19 patients and this line of investigation has largely been abandoned.
A probable explanation for HIV protease inhibitor failures in COVID-19 treatment is their high specificity for the HIV- encoded aspartic protease, whereas coronaviruses encode two cysteine proteases. Protease inhibitors are, however, a highly successful class of antiviral drugs, and both SARS-CoV-2 proteases are considered attractive drug targets as their activity is essential for virus replication. Previous extensive drug discovery efforts targeting SARS-CoV/ MERS-CoV proteases, combined with the rapid elucidation of the X-ray structure of both SARS-CoV-2 proteases, provides an excellent starting point that is driving a large research effort to develop SARS-CoV-2 protease inhibitors, with multiple lead compounds under active investigation.
In addition to the key RNA-dependent RNA polymerase and protease enzymatic targets, multiple other steps in the SARS-CoV-2 replication cycle are being intensively investigated as potential drug targets. The research effort ranges from highly targeted approaches, such as those briefly discussezd above, to high-throughput screening of large compound libraries. A robust drug discovery research pipeline is essential as there is no guarantee that leading drug candidates currently in advanced clinical trials will ultimately yield a positive outcome.
Safety and efficacy are essential criteria, but several other challenges must be overcome. Time of antiviral treatment is critical and should be as early as possible to halt virus replication before disease progression, as the short therapeutic window for treatment of acute respiratory viruses limits antiviral effectiveness. Ideally, the antiviral drug should be in the form of an oral pill, which can be administered in a variety of settings. Currently, remdesivir can only be given in hospital, as it is administered intravenously due to its poor bioavailability and short half-life, limiting its usefulness. Acquisition of drug resistance is a widely documented challenge in the use of antiviral drugs against RNA viruses and is typically overcome via combination therapy consisting of two or more antiviral drugs with different mechanisms of action.
Antiviral drug discovery and development is expensive and time consuming. However, the magnitude of the COVID-19 pandemic is sufficient to drive the global research effort, financial investment and political will required to deliver effective antiviral treatments with accelerated clinical testing and rapid manufacturing scale-up. For example, in April 2021 the UK government launched the COVID-19 Antivirals Taskforce, which aims to identify at least two effective treatments, either in tablet or capsule form, that the public can take at home to combat any future increase in infections and limit the impact of new viral genetic variants. The stated timeline is rollout by the end of 2021; it remains to be seen if this ambitious goal can be achieved.
Therapeutic antibodies targeting SARS-COV-2 are being rapidly developed for COVID-19 treatment. The starting point for using neutralising antibodies (nAbs) to treat COVID-19 is the use of convalescent plasma, donated by patients who have survived SARS-CoV-2 infection by producing these protective antibodies. Although the use of convalescent plasma has received an FDA EUA, clinical trial data has been disappointing, along with difficulties associated with standardising the potency of plasma doses. Instead, the development of monoclonal antibodies (mAbs) has proven more advantageous in COVID-19 treatment.
Several mAbs have been rapidly developed and all target the SARS-CoV-2 spike protein, which mediates virus entry via interaction with host cell receptor angiotensin-converting enzyme 2 (ACE2). The vast majority of these mAbs target epitopes within the receptor-binding domain (RBD) of the spike protein. The principal mechanism of virus neutralisation is blocking infection of target cells by antibody binding to the viral spike protein and preventing ACE2 interaction.
Clinical investigation of mAbs has thus far resulted in a small number being granted FDA EUA; at the current time, FDA EUAs exist for REGEN-COV, a combination of two mAbs administered together (casirivimab and imdevimab), and sotrovimab, administered as a monotherapy. Clinical trials are investigating mAbs’ effectiveness in different patient scenarios; however, existing EUAs are for the treatment of mild–moderate COVID-19 patients who are at high risk for progressing to severe COVID-19 and/or hospitalisation.
A second mAb combination therapy (bamlanivimab and etesevimab) had its FDA EUA paused in June 2021, due to evidence that both mAbs are not active against certain circulating SARS-CoV-2 genetic variants of concern (VOC). Indeed, several mutations found in VOC have been shown to abolish the neutralisation activity of multiple mAbs. Monitoring the resistance of mAbs to circulating variants will be critical for their therapeutic use and will guide future development strategies.
Targeting the host
The severity of COVID-19 depends on many factors, but a common hallmark of severe disease is dysregulation of the immune/inflammatory response. Immune factors can be impaired or downregulated; a key example is the interferon (IFN) response, a vital part of the innate immune response to counteract virus spread in the early stages of infection. Elevated levels of several proinflammatory cytokines, often referred to as a cytokine storm, is also a signature of severe COVID-19. The consequence of this immune dysregulation is robust virus regulation and inflammatory damage to tissues within the body, both of which are linked to disease severity. Host-targeted strategies are required to relieve the symptoms caused by dysregulation of the immune/inflammatory response, and repurposing of existing immunomodulatory and anti-inflammatory agents has been utilised for the discovery of host-directed COVID-19 treatments.
One therapeutic strategy is the replacement of host factors that have been impaired or downregulated during infection. Administration of purified IFN has been used in the clinic against several viruses, most notably hepatitis C virus (HCV), on the rationale that treatment with this vital cytokine will stimulate the IFN signalling pathway, activating expression of IFN-stimulated genes (ISGs) to induce an antiviral state to block virus infection and spread within the body. Multiple clinical trials are being evaluated for the effect of IFN treatment in COVID-19 patients. To date, the results are mixed and indicate that IFN treatment may only be beneficial for a subset of patients with a minimal IFN response that are treated early in infection.
Another strategy is inhibition of COVID-19 elevated cytokines. IL-6 is one of the signature cytokines that is excessively upregulated in the cytokine storm. Treatment with a mAb (tocilizumab [Actemra]) which binds to the IL-6 receptor, inhibiting IL-6 mediated signalling, has recently been granted an FDA EUA for use in certain hospitalised COVID-19 patients. The Janus kinase 1 and 2 (JAK 1/2) inhibitor baricitinib (Olumiant) in combination with remdesivir also has an FDA EUA for treatment of certain hospitalised COVID-19 patients. JAK 1/2 signalling plays an important role in the cytokine-mediated host inflammatory response to infection and disease.
Early in the pandemic, anti-parasitic drugs chloroquine and hydroxychloroquine were tested as potential COVID-19 treatments. The mechanism of action of these drugs is not clearly understood, but antiviral and immunomodulatory activities have been proposed. In June 2020 the FDA revoked the EUA for these drugs on the basis that they are unlikely to be effective in treating COVID-19, together with ongoing serious side effects.
Dexamethasone is a corticosteroid that is widely used for its anti-inflammatory and immunosuppressant effects. In June 2020 the RECOVERY trial announced that dexamethasone reduces death by one third in hospitalised patients with severe respiratory complications of COVID-19. Given that dexamethasone is off-patent, cheap and widely available, it provides the capacity for a global treatment option, which is already being translated into saving lives.
Summary
The COVID-19 pandemic has brought into sharp focus our lack of treatments that can be rapidly deployed upon emergence of a new pathogenic virus. A remarkable global research effort is underway to discover, develop, test and manufacture new treatments against COVID-19. The continued success of this research effort is dependent upon sustained financial investment together with coordinated political will to tackle the current COVID-19 pandemic, but also so that we are better prepared for the emergence of the next deadly virus.
Further reading
Adamson CS, Chibale K, Goss RJM, Jaspars M, Newman DJ et al. Antiviral drug discovery: preparing for the next pandemic. Chem Soc Rev 2021;50:3647–3655.
Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell 2021;184:3086–3108.
Heutess AM, Allard M, Thompson DK, Fasinu PS. Clinical management of COVID-19: a review of pharmacological treatment options. Pharmaceuticals 2021;14:520
Lowery SA, Sariol A, Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: implications for COVID-19. Cell Host Microbe doi.org/10.1016/j.chom.2021.05.004 [Epub ahead of print].

Jane Hilton
School of Biology, Biomedical Sciences Research Complex, University of St Andrews, North Haugh, St Andrews, Fife KY16 9ST, Scotland, UK
Jane Hilton undertook her PhD in Neuroscience at the University of St Andrews, before transitioning into industry and advancing as a screening scientist in Drug Discovery. In 2020, Jane moved back to academia, working in Dr Catherine Adamson’s lab to contribute to the fight against coronavirus at the University of St Andrews.

Catherine S. Adamson
School of Biology, Biomedical Sciences Research Complex, University of St Andrews, North Haugh, St Andrews, Fife KY16 9ST, Scotland, UK
Catherine Adamson is Senior Lecturer in Molecular Medicine in the School of Biology at the University of St Andrews. Catherine’s area of research expertise is antiviral strategies against viruses including HIV and RSV. Catherine is currently the virology lead on multiple interdisciplinary projects to combat SARS-CoV-2/Covid-19. Catherine has been a member of the Microbiology Society for 28 years.
What do you love most about your job?
Catherine: First and foremost, I love biology and science in general. I enjoy the huge variety of work that I conduct on a daily basis and the opportunity to interact with a diverse range of talented colleagues. The real joy of the job comes from learning something new almost every day.
Jane: I feel privileged to work on SARS-CoV-2 in a BSL3 facility and to be part of a multidisciplinary team of physicists and biologists working together to meet the challenge of identifying novel engineered surfaces that could potentially be used to supress the transmission of SARS-CoV-2 in public spaces.
How did you enter this field?
Catherine: I became interested in virology as an undergraduate student. At that time, during the 1990s, we were in the midst of the HIV/AIDS pandemic. I conducted doctoral and postdoctoral research into HIV, and now as Principal Investigator I am conducting research that will contribute to tackling the 2nd major viral pandemic that has affected humanity in my lifetime, the SARS-CoV-2/COVID-19 pandemic.
Jane: During the COVID-19 pandemic, an opportunity became available to work in Dr Catherine Adamson’s lab on SARS-CoV-2; I was already interested in virology and the research that was being conducted in Dr Adamson’s lab. As a scientist, I was keen to be involved and utilise my skills and experience to contribute to the field of SARS-CoV-2 research.Image: iStock/Lacheev.
