Antimicrobial-resistant gonorrhoea - the ongoing challenge to ensure gonorrhoea remains a treatable infection
Issue: Sexually transmitted infections (STIs)
21 May 2013 article
The control of bacterial STIs for public health is dependent on delivery of prevention messages to raise awareness, use of appropriate diagnostic tests to reduce the burden of infection and provision of effective antimicrobial therapy to break transmission. In the absence of an apparent protective immune response, and hence no effective vaccine, antimicrobial therapy is an essential component of control but is often compromised by resistance resulting in therapeutic failure.
CATHERINE ISON
Bacterial STIs are often treated on clinical presentation (syndromically) or, if laboratory testing is performed, before results are available. Therapy with a single dose of an antimicrobial, to which resistance rates are not known or are <5%, has been favoured with the aim of achieving >95% therapeutic success. National, regional and global surveillance data help inform the choice of antimicrobial but, especially for gonorrhoea, these data are limited and the same or similar regimens are recommended in many different countries.
Strengthening our prevention measures, particularly for at risk groups, is essential to reduce the burden of gonorrhoea. It is paramount that the versatility of N. gonorrhoeae is never underestimated.
The challenge
The effective treatment of gonorrhoea has been a challenge because of the propensity of Neisseria gonorrhoeae, the infecting organism, to become resistant to successive antimicrobial agents over the last five decades. This genetically diverse bacterium is capable of acquiring DNA for recombination at all stages of its life cycle. Many strains of N. gonorrhoeae have acquired plasmids from strains of Haemophilus and Streptococcus that confer high-level resistance to penicillin and tetracycline, respectively, and chromosomal DNA from commensal neisseriae conferring resistance to penicillin. Selection of resistant strains can also occur and can be the result of inadequate dosage, the use of drugs available over the counter, often lacking full potency, and long-term use of a single agent, as has been the practice in the treatment of many STIs. In some strains of N. gonorrhoeae, selection of high-level resistance to spectinomycin and azithromycin has occurred in a single step and low-level resistance to penicillin has emerged by the cumulative effects of multiple mutations.

Past therapies
The first antimicrobial agent used for the treatment of gonorrhoea was sulphonamide in 1937 but resistance quickly emerged and so this treatment was replaced with penicillin. After many decades with penicillin as the treatment of choice, albeit at ever-increasing dosages, resistance reached >5% due to high-level plasmid-borne and low-level chromosomally acquired resistance and it was replaced by the highly active fluoroquinolone ciprofloxacin. At the time of its introduction, resistance to ciprofloxacin in N. gonorrhoeae was not documented and the drug was extremely effective, resulting in the temptation to use low doses. A single oral dose of 250mg was recommended but there were multiple reports of the use of 125mg or even 62.5mg. This practice, together with the use of over-the-counter and early-generation quinolones in some parts of the world, led to the selection of mutants with increasing levels of resistance over time, limiting its useful life span even at higher doses, such as 500mg.
Emerging problem
The third generation cephalosporins, cefixime and ceftriaxone, eventually replaced ciprofloxacin as resistance levels soared; in the UK, this occurred in 2004. Cefixime, an oral agent, was preferred due to its ease of administration, whereas ceftriaxone, an injectable, was used for pharyngeal infections because it was known to penetrate this site more effectively than cefixime. Resistance and treatment failure were not documented in N. gonorrhoeae at that time, although past events should have been a warning to the scientific and medical community that it was only a matter of time before this would occur again. The first report of treatment failure to cefixime was in Japan in 2001, probably resulting from the use of a number of other oral cephalosporins, some at suboptimal doses. Further reports of treatment failure were slow to appear but this may have been an artefact, in part, due to the widespread use of molecular testing for diagnosis and the challenge of verifying treatment failure without identifying the infecting organism. Subsequently, treatment failure to cefixime was documented in a number of countries with the first reports in the UK in 2011, although the true prevalence is unknown and was almost certainly greater than is evident.
Surveillance programmes began to describe decreased susceptibility to cefixime but in the absence of sufficient data from treatment failures, the definition of resistance was unclear. This is of concern, particularly in the case of the emergence of a bimodal population, suggesting the establishment of a ‘resistant’ population. Decreased susceptibility to cefixime has been found to be associated with the acquisition of mosaic penA genes, which alter the target site for penicillin and cephalosporins in penicillin binding protein 2 (PBP2). It is thought that these genes may have been acquired from commensal neisseriae present in the throat during pharyngeal gonococcal infection. Mutations in other genes affecting penicillin resistance, such as mtr (efflux) and penB (porin), have also been implicated but appear to play only a minor role, although full levels have not been replicated in the laboratory, suggesting the presence of an, as yet, unknown factor X. Molecular typing studies indicate that this drift towards decreased susceptibility is clonal and likely to have originated in Japan but is now the predominant strain in many countries and found widely in Europe. In some countries, such as the UK, infection has been found predominantly among men who have sex with men (MSM) but heterosexual infection has also been detected in some countries and may just reflect circulation in different sexual networks rather than a preference of this strain for any particular anatomical site.
Innovative response
Concern began to mount in 2011 that this was the beginning of the end of the useful life of third-generation cephalosporins for treatment of gonorrhoea and consideration was given as to whether a change in therapeutic agent was necessary. There was no precedent for changing therapy before it became <95% effective, as recommended by the World Health Organization, but the potential for gonorrhoea to be difficult or impossible to treat appeared to be becoming a reality. The lack of suitable alternative agents to which N. gonorrhoeae was susceptible was limited and left few options for treatment, including use of combinations of drugs, a return to agents used previously such as gentamicin or longer courses of treatment. In the first instance, ceftriaxone, believed to be the more active drug, was the obvious choice as emergence of decreased susceptibility had been slower than for cefixime and only occasional reports of treatment failure had occurred. Also, serious concerns were raised about the logistical problems associated with widespread use of an injectable and the effect this had on compliance, but choices were limited. The recommended dose of ceftriaxone was also controversial as pharmacokinetic data suggested that if a 250mg dose were to be used, this would likely be useful for only a short time. Higher doses of 500mg or 1g are licensed for use and give a greater concentration of the drug and persist for a longer time in the bloodstream, giving the bacterium a greater challenge to overcome before this treatment too would become insufficient.
Decreased susceptibility to cefixime has been found to be associated with the acquisition of mosaic penA genes, which alter the target site for penicillin and cephalosporins in penicillin binding protein 2 (PBP2).
These concerns resulted in timely changes to guidelines, most recommending ceftriaxone at an increased dose of 500mg in combination with azithromycin at 1 or 2g, intended to treat any concomitant Chlamydia infection, but also to give additional cover for gonorrhoea if, or when, decreased susceptibility emerged. In 2012/2013, plans were published at a global, national and regional level to raise awareness and give advice on combating the threat of antimicrobial-resistant gonorrhoea and, most importantly, giving guidance on the definition of a probable or confirmed treatment failure.
The future
The response to the threat of antimicrobial-resistant gonorrhoea has been prompt, despite some criticism of scaremongering. The emergence of decreased susceptibility appears to have been arrested in many countries, although this is balanced by recent reports of therapeutic failure due to multi-drug-resistant strains, often reported as ‘superbugs’. It is impossible to attribute the decline in resistance entirely to the innovative approach taken; it is highly probable that this has delayed rather than reversed the problem but this has bought time for new drugs to be developed and different approaches to treatment to be put through clinical trials. The future strategy needs to be multifaceted and should include the appropriate use of new diagnostic tests and retention of expertise in the isolation of viable organisms to enable both the detection of emerging resistance and the provision of timely surveillance data. Strengthening our prevention measures, particularly for at risk groups, is essential to reduce the burden of gonorrhoea. It is paramount that the versatility of N. gonorrhoeae is never underestimated. The challenge is to ensure that gonorrhoea remains a treatable infection.
CATHERINE ISON
Head, Sexually Transmitted Bacteria Reference Unit, Microbiological Services, Public Health England, 61 Colindale Avenue, London NW9 5EQ; Tel. 020 8327 6462; Email [email protected]
Further reading
Bolan, G.A., Sparling, P.F. & Wasserheit, J.N. (2012). The emerging threat of untreatable gonococcal infection. N Engl J Med 366, 485–487.
Chisholm, S.A., Mouton, J. W., Lewis, D. A., Nichols, T., Ison, C.A. & Livermore, D.M. (2010). Cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink? J Antimicrob Chemother 65, 2141–2148.
ECDC (2012). Response plan to control and manage the threat of multidrug resistant gonorrhoea in Europe. Stockholm: European Centre for Disease Prevention and Control. www.ecdc.europa.eu/en/publications/Publications/Forms/ECDC_DispForm.aspx?ID=901 (last accessed 25 March 2013).
Health Protection Agency (2013). Gonococcal Resistance to Antimicrobials Resistance Programme (GRASP) Action Plan. www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1317137983229 (accessed 25 March 2013).
Lewis, D.A. (2010). The gonococcus fights back – is this time a knock out? Sex Transm Infect 86, 415–421.
WHO (2012). World Health Organization Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/9789241503501/en/index.html (last accessed 25 March 2013)
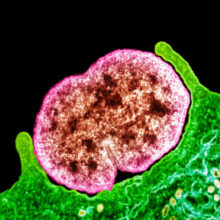
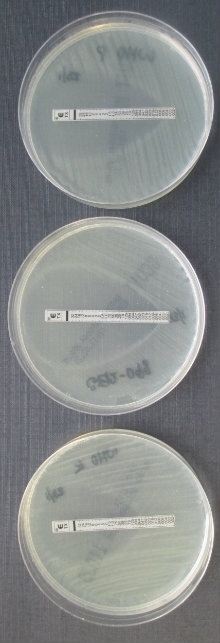
Image: Fig. 1. Coloured transmission electron micrograph of N. gonorrhoeae bacteria diplococci (pairs of cells, pink) infecting a human epithelial cell (green). Science Photo Library Fig. 2. Albert Neisser (1855–1916), the German physician who discovered and gave his name to N. gonorrhoeae. National Library of Medicine / Science Photo Library Fig. 3. Use of E-test strips to measure the resistance of bacterial cultures to different antibiotics. C. Ison.
