Pregnancy, the placenta and Zika virus (ZIKV) infection
Issue: The Mobile Microbe
08 November 2016 article

Zika virus (ZIKV) infections have been recognised in Africa and Asia since 1940. The virus is in the family Flaviviridae and genus Flavivirus, along with Dengue, Japanese encephalitis virus, Tick borne encephalitis, West Nile virus, and Yellow fever virus. These viruses share biological characteristics of an envelope, icosahedral nucleocapsid, and a non- segmented, positive sense, single-strand RNA genome of ~10 kb encoding three structural proteins (capsid C pre-membrane/membrane PrM/M, envelope E), and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5). ZIKV has three known genotypes; the West African (Nigerian cluster), East African (MR766 prototype cluster), and Asian strains. Virus sequencing from the most recent South American outbreak suggests this virus is related to the 2013 French Polynesian isolates of Asian lineage.
ZIKV like other flaviviruses is arthropod-borne (arbovirus), with more recent evidence for sexual transmission, persistent presence in semen1, and higher rates of acquisition due to a higher reproductive number than Dengue virus (DENV)2. Infection with ZIKV is usually asymptomatic (~80% of cases) or causes mild disease similar to less severe DENV. However, ZIKV has emerged as a major public health threat globally due largely to substantial recent outbreaks in areas of large gatherings, and observed association with fetal neurological damage including microcephaly in the Americas and elsewhere (reviewed in Marrs et al.1). Countries involved in the most recent outbreaks are summarised in the associated paper here. As a result of the risk to pregnant women, Australian public health authorities (and those in many other countries) recommend pregnant women defer travel to high risk countries. However, if exposure is likely, these women should prevent mosquito bites, have their sexual partners avoid mosquito bites, and post exposure avoid pregnancy for 8 weeks (summarised in Marrs et al.1), or possibly longer.
Clinical outcomes of mother to child transmission and diagnostic difficulties
Mother to child transmission (MTCT) of ZIKV has been documented via placental infection and damage, with increasing evidence of fetal ZIKV microcephaly. The number of infected mothers, compared with number of infected fetuses (i.e. rate of MTCT), is unclear, although in one Brazilian study, of 88 pregnant women with rash before 38 weeks gestation, 82% had ZIKV and 12/42 (27%) had fetal abnormalities on ultrasound compared with 0/16 women without ZIKV3. However, this is likely a significant overestimate due to the method of collection, the nature of the clinic and the lack of confirmed transmission on amniocentesis. A case control study of association between ZIKV and microcephaly showed ZIKV present in mothers of 24/32 cases of microcephaly compared with 39/61 mothers of controls (p = 0.12), and that in the babies, 13/32 with microcephaly compared with 0/16 of the controls had ZIKV infection4. These rates compare with rates of MTCT in maternal cytomegalovirus (CMV) infection of 32% in primary infection and 1.4% during reactivation5, and for rubella of 80% to 25%, depending upon gestation6. Effects on the fetus for all these infections depend upon many factors, including maternal immunity, gestation of infection, and viral characteristics.
Identification of mothers infected with ZIKV is predominantly via symptoms, serology, and molecular testing of the acutely infected person. Diagnosis is confounded by the low rate of symptoms (in ~20% of adults), technical difficulties with serology cross- reactivity, and the brief period of viraemia in some infections. Serology diagnostic problems occur due to co-circulation of other flaviviruses (particularly Dengue virus) in ZIKV affected areas. Cross-reactivity between ZIKV and Dengue virus occurs7, falsely negative tests for ZIKV may result if high levels of antibody are present to other flaviruses (such as occurs following vaccination for Yellow fever virus), and acute ZIKV infection may result in false positive Dengue NS1 antigen tests8, further confusing diagnosis. Molecular testing using nucleic acid tests such as PCR is definitive if positive, although the duration of viraemia makes identification difficult when combined with low rates of symptomatic infection.
A major concern is whether MTCT occurs in ZIKV-infected asymptomatic women resulting in unexpected fetal damage. This occurs in murine models where ZIKV tropism for cells at the maternal-fetal interface is the likely source of transplacental transmission9, and is consistent with human cell studies in vitro10. Prolonged maternal viraemia, and excretion of ZIKV in urine for 5–6 weeks following infection provides opportunities for improved diagnosis11, but also the possibility of continuing risk of ZIKV transmission either to other adults or MTCT during asymptomatic phases of an infected mother1. ZIKV has been found in breast milk in three case reports of mothers infected <3 days from delivery12, although MTCT transmission via breast milk has not been documented.
Placental and fetal infection
Most microcephaly is thought to arise from first trimester (T1) infection, although sampling difficulties occur with the high rate of asymptomatic infection. ZIKV has been detected in fetal brain tissue from microcephalic infants, in amniotic fluid taken from mothers of affected infants13, and from central nervous system tissue of affected microcephalic infants14. These are mainly observational data with minimal controls, albeit with autopsy and ultrasound data being consistent with microcephaly resulting from ZIKV infection during pregnancy15. ZIKV has been known to be neurotropic in animals for 60 years, with more recent murine experiments demonstrating replication in embryonic brain targeting neural progenitor cells, with consequent cell cycle arrest, apoptosis and inhibited neural progenitor cell differentiation9,16. This is presumed to result in the microcephalic phenotype via neuronal cell death16. This is consistent with observations that African ZIKV strains infect neural precursor cells in murine models (summarised in Klase et al.17).
Mother to child transmission (MTCT) studies often use models from T2 or T3 placentae, which differ from T1 placentae in structure, cell components and surface markers. Studies of infection of explanted placentae in other viral infections such as with CMV show neonatal neural malformation and intra uterine death may be caused partly through Th1 cytokine-induced placental damage18,19. The placenta is a complex organ that changes significantly over pregnancy, and comprises some unique cells with differential susceptibility to viral infection (Figure 1). ZIKV infects isolated placental primary cells and human placentae cultured ex vivo, with mid pregnancy (T2) chorionic villi (cytotrophoblasts, endothelial cells, fibroblasts, Hofbauer cells) and amniochorionic membranes (amnion epithelial cells, trophoblast progenitors) infected10. As MTCT requires virus to traverse the placenta, the role of trophoblasts (either as differentiated syncytiotrophoblasts or cytotrophoblasts) is likely to be key, similar to the key role they have for MTCT of other viruses18. Placental inflammatory response to ZIKV may be important in fetal neurological pathology, although this remains to be proven in humans.
FIGURE 1. POSSIBLE SITES OF ZIKA VIRUS INFECTION OF THE HUMAN PLACENTA.
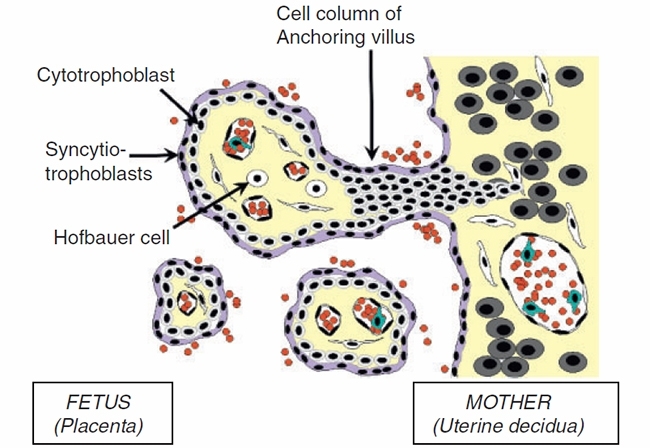
Early gestation (T1) infection with ZIKV has been associated with miscarriage, intrauterine growth restriction, and microcephaly14, and although causation is likely, it is still to be proven. These changes result from direct infection of the fetal neuronal tissue, although placental infection may contribute to the more generalised fetal pathology as occurs with other viruses causing similar fetal pathology, possibly through virus-induced cell cycle dysregulation20. The presence of receptors and cell entry cofactors on these cells (Axl, Tyro3, TIM1) which are known also to be bound by other flaviviruses (DENV – Tyro3, Axl, Mertk) suggest a common mechanism of entry may exist21. These receptor tyrosine kinases are from a family known to clear apoptosed cells and interact with the innate immune system. Interventions that prevent ZIKV binding to these may provide therapeutics that can be trialled in mouse models or human placental explant models where reduced placental damage may reduce fetal injury9.
Future studies
ZIKV infection remains a disease clinically of either no symptoms, or relatively mild presentation with fever, myalgia, eye pain, and/or fatigue associated with a maculopapular rash. The major complication of fetal injury, particularly microcephaly and death in utero, need to be addressed with further research. Good murine and human placental explant models exist18, and candidate targets for ZIKV cell binding inhibition have been identified10. Vaccines for related flaviviruses are now licensed in some countries (DENV – the live recombinant Denvaxia from Sanofi Pasteur) or undergoing trials. If continued spread of ZIKV occurs either within currently infected countries, or to other naïve populations, enhanced vaccine development needs to be considered, as suggested by some commentators. If so, such a vaccine will need to prevent MTCT and address the issue of cytokine/immune-dependent injury to the fetus, transplacental transmission of ZIKV10,18, with the potential to significantly reduce the risk of congenital ZIKV abnormalities, as has occurred with the successful use of vaccines for rubella virus. Finally, all sources of ZIKV transmission to pregnant women should be avoided, including via blood products, as these may be infected despite being from asymptomatic individuals22.
Acknowledgements
Thanks to Stuart Hamilton for the figure, and to Stuart Hamilton and Wendy van Zuylen for manuscript review.
WILLIAM RAWLINSON
Serology and Virology Division, SEALS Pathology, Level 4, Clinical Sciences Building, Prince of Wales Hospital, Randwick, NSW 2031, Australia
[email protected]
REFERENCES
- Marrs, C. et al. (2016) Zika virus and pregnancy: a review of the literature and clinical considerations. Am. J. Perinatol. doi:10.1055/s-0036-1580089
- Bastgos, L. et al. (2016) Zika in Rio de Janeiro: assessment of basic reproductive number and its comparison with dengue. bioRxiv 055475. doi:10.1101/055475
- Brasil, P. et al. (2016) Zika virus infection in pregnant women in Rio de Janeiro – preliminary report. N. Engl. J. Med. doi:10.1056/NEJMoa1602412
- Araujo, T.V.B. et al. (2016) Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case- control study. Lancet Infect. Dis. doi:doi:S1473-3099(16)30318-8
- Scott, G.M. et al. (2012) Cytomegalovirus infection during pregnancy with materno-fetal transmission induces a pro-inflammatory cytokine bias in placenta and amniotic fluid. J. Infect. Dis. 205, 1305–1310. doi:10.1093/infdis/jis186
- Dontigny, L. et al. (2008) Rubella in pregnancy. J. Obstet. Gynaecol. Can. 30, 152–158. doi:10.1016/S1701-2163(16)32740-2
- Lanciotti, R.S. et al. (2008) Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 14, 1232–1239. doi:10.3201/eid1408.080287
- Gyurech, D. et al. (2016) False positive dengue NS1 antigen test in a traveller with an acute Zika virus infection imported into Switzerland. Swiss Med. Wkly. 146, w14296.
- Miner, J.J. et al. (2016) Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091. doi:10.1016/j.cell.2016. 05.008
- Tabata, T. et al. (2016) Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 20, 155–166.
- Gourinat, A.C. et al. (2015) Detection of Zika virus in urine. Emerg. Infect. Dis. 21, 84–86. doi:10.3201/eid2101.140894
- Colt, S. et al. (2016) Transmission of Zika virus through breast milk and other breastfeeding-related bodily fluids: a systematic review. Bull. World Health Organ. 2, doi:10.2471/BLT.16.176677
- Calvet, G. et al. (2016) Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect. Dis 16, 653–660. doi:10.1016/S1473-3099(16)00095-5
- Mlakar, J. et al. (2016) Zika virus associated with microcephaly. N. Engl. J. Med. 374, 951–958. doi:10.1056/NEJMoa1600651
- Broutet, N. et al. (2016) Zika virus as a cause of neurologic disorders. N. Engl. J. Med. 374, 1506–1509. doi:10.1056/NEJMp1602708
- Li, C. et al. (2016) Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19, 120–126. doi:10.1016/j.stem.2016.04.017
- Klase, Z.A. et al. (2016) Zika fetal neuropathogenesis: etiology of a viral syndrome. PLoS Negl. Trop. Dis. 10, e0004877. doi:10.1371/journal.pntd.0004877
- Hamilton, S.T. et al. (2012) Human cytomegalovirus induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One 7, e52899. doi:10.1371/journal.pone.0052899
- Hamilton, S.T. et al. (2013) Human cytomegalovirus directly modulates expression of chemokine CCL2 (MCP-1) during viral replication. J. Gen. Virol. 94, 2495–2503. doi:10.1099/vir.0.052878-0
- van Zuylen, W.J. et al. (2016) Human cytomegalovirus modulates expression of noncanonical Wnt receptor ROR2 to alter trophoblast migration. J. Virol. 90, 1108–1115. doi:10.1128/JVI.02588-15
- Meertens, L. et al. (2012) The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 12, 544–557. doi:10.1016/j.chom.2012.08.009
- Musso, D. et al. (2014) Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 19, 20761. doi:10.2807/1560-7917. ES2014.19.14.20761
Image: Figure 1. Cytotrophoblasts (CTB) form the inner layer of villi, fuse into multinucleated syncytiotrophoblasts, or give rise to extravillous trophoblasts (EVT), which invade and migrate into maternal uterine decidua (that is from left to right in the figure). Viruses infect different cell types, with ZIKV shown in ex vivo explants to infect CTB, endothelial cells, fibroblasts, and Hofbauer macrophage-like cells in the villus coreon the uterine decidual side (Tabata et al.10)..
