Legions of water-borne bacterial diseases
Issue: Water
17 November 2014 article
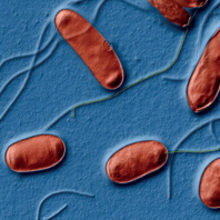
In 1976, the discovery of antibiotics and the use of vaccination programmes supported a belief that infectious diseases had largely been understood and overcome.
1976 held a special significance in the USA as the country celebrated the bicentennial anniversary of the signing of the 4 July Declaration of Independence. More than 2,000 members of the Pennsylvanian American Legion of war veterans celebrated this historic event during their annual three-day convention at the Bellevue-Stratford Hotel, Philadelphia on 21–23 July. Then nearly two weeks later, the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia was alerted to the deaths of four veterans who had died from suspected pneumonia after attending the convention. Subsequently, with an incubation period of 2–10 days, a cluster of cases followed with reported symptoms of a mild cough, fever and, in some people, a progressive pneumonia that led to death. By the end of the epidemic, 182 members of the legion were diagnosed and 29 fatalities reported. In addition, another 39 people who had been in the close vicinity of the hotel developed a similar disease that caused another five deaths.
In many ways this outbreak marked a turning point in disease surveillance as it became apparent that ‘new’ infectious diseases would continue to be identified and a state of readiness was vitally important. The CDC began to track down the cause of this infection. The key risk factors for the illness were found to include old age, being male and being a smoker, as well as spending time in the lobby or outside the front door of the hotel. There was negligible evidence to suggest the disease was associated with food or person-to-person spread and it became clear that this was an air-borne infection. However, it was nearly six months after the outbreak before the perpetrator of the disease was identified, the bacterium Legionella pneumophila.
Identifying the disease
Tantalising clues about the cause of the disease had emerged. For example, in laboratory tests using eggs inoculated with infected lung tissue from the diseased victims, several of the eggs died because of so-called ‘bacterial contamination’. Also, guinea pigs that were inoculated with infected human tissue became ill, but the diseased guinea pig tissue could not transmit the infection to other guinea pigs.
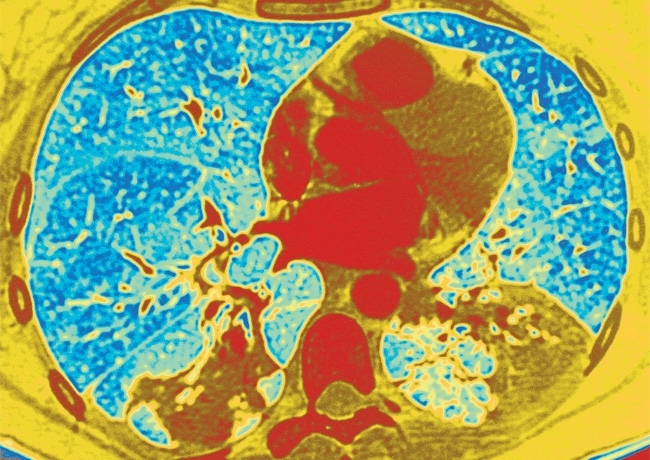
Joseph McDade was the scientist whose initiative, persistence and previous experience with Rickettsia allowed him to follow the clues. In late December 1976, McDade decided to re-examine slides, stained for bacteria, taken from guinea pigs that had died after he had inoculated them with diseased material earlier that year. Eventually he spotted a small cluster of Gram-negative bacilli. Rather than dismissing them as bacterial contamination, he isolated them as if they were the Rickettsia. The confirmation that these were the disease-causing bacteria came when the sera of patients from the Philadelphia outbreak gave a positive antibody-mediated response to the isolated bacteria. Legionnaires’ disease and its newly associated pathogenic bacterium, L. pneumophila, had been found.
Legionella, a new bacterium?
L. pneumophila was quickly associated with a raft of subsequent outbreaks of community- and hospital-acquired pneumonia. However, it also provided fresh insight into previous disease outbreaks of unknown origin, including an outbreak of a respiratory disease that had affected workers and visitors to a health department in Pontiac, Michigan, USA, eight years previously. Unlike the symptoms of Legionella, the subsequently named Pontiac fever is an acute self-limiting disease with a short incubation period and flu-like symptoms (Table 1). To date, it is still not clear why infection with L. pneumophila causes two distinct syndromes as a result of inhalation of contaminated aerosols produced by man-made water systems, including cooling-towers, showers, air-conditioning systems, hot tubs, and occasionally through direct placement of L. pneumophila into the lungs during respiratory tract manipulations.
TABLE 1. COMPARISON OF THE TWO SYNDROMES ASSOCIATED WITH L. PNEUMOPHILA INFECTION
|
Legionnaires’ disease |
Pontiac fever |
|
Mild cough to fatal pneumonia. Death is an outcome of respiratory, kidney and/or multi-organ failure |
Acute self-limiting influenza-like illness. Symptoms usually last 2–5 days but the disease is not fatal. |
|
Incubation period: 2–16 days |
Incubation period: up to 48 hours |
|
Symptoms: fever, anorexia, headache, lethargy, muscle pain, diarrhoea and confusion. Blood-streaked phlegm can also occur in some patients. |
Symptoms: fever, chills, headache, sore muscles and joints.
|
So where had this infection come from?
L. pneumophila is just one of more than 50 species and 70 serogroups of Legionella that have since been isolated. Only a small fraction of these species cause human infection, but this includes Legionella longbeachae, a soil-borne pathogen that causes disease through exposure to aerosols formed from commercial potting compost. Legionella are Gram-negative, non-spore-forming, flagellated bacilli, which are ubiquitous in freshwater systems, including lakes, rivers and thermal springs. However, Legionnaires’ disease is not associated with exposure to the bacteria from these natural environments. It is man-made water systems with a temperature range between 20 and 42°C that provide favourable conditions for bacterial growth. The stagnant water and low water pressure associated with hotels, ferries and cruise ships has also ensured that travel has been identified as a risk factor for Legionnaires’ disease. Legionella have been shown to survive temperatures of 54°C, and below 20°C as the bacteria hibernate while they wait for conditions that are more favourable for growth.
Legionella as parasites
The life of bacteria in the environment is very different to their cultured laboratory life. Microbes can exist in assembled, complex communities with shared survival mechanisms. These include essential intracellular communications, which are key for the survival of microbes in environments such as domestic water supply systems that are low in nutrients. Biofilms develop on surfaces of stagnant or undisturbed non-sterile water and have been shown to be the concentrated source of micro-organisms in this environment. Legionella form part of these water-borne biofilms that can be notoriously hard to remove from the surfaces of man-made water systems. These biofilms can also contain a large variety of protists that include amoeba and ciliated protozoa.
A large number of free-living protozoa have been shown to host L. pneumophila, providing ready access to a range of free nutrients that allow Legionella to obtain the nutrients and energy supply they need to replicate. Legionella that have been associated with other organisms, such as protists, have been found to be more pathogenic to humans. When the bacteria are engulfed or phagocytosed by the protozoa they are housed within the Legionella-containing vacuole (LCV). An elegant survival trick by the bacteria is that they ensure the LCV manages to avoid being targeted by the intracellular lysosomes that contain the hydrolytic acidic environment that usually degrades and removes engulfed invasive bacteria. Instead, the bacteria use mechanisms that allow the cellular LCV to change its membrane structure, disguising itself as part of the normal cellular organelle, the endoplasmic reticulum. This ensures that the LCVs that contain the bacteria avoid being targeted by the cellular lysosomes.
Interestingly, the mechanisms that Legionella employ to evade destruction in humans are similar. The bacteria replicate in LCVs contained in the alveolar macrophages where they evade destruction, but also convert the normal cellular processes of the macrophages to produce short peptides and amino acids. Legionella convert cysteine and serine to pyruvate, which is fed into the TCA cycle to generate the major sources of carbon and energy required by the bacteria to survive and replicate. The long association between Legionella and protozoa has led to the transfer of a suite of advantageous genetic elements to the bacteria. These have enabled the bacteria to acquire effector molecules that allow them to adapt to the intracellular environment of the protozoa. Subsequent man-made manipulation of the natural environment generated new water systems that allowed aerosols of these bacteria, including L. pneumophila, to be generated and inhaled. This in turn allowed the bacteria to use the skill set it acquired from cohabitation with protists to adapt to life within human phagocytes. Once inside human cells L. pneumophila is able to delay apoptosis (programmed cell death) of the macrophages, allowing the bacteria to proliferate within the cell before they are released to infect other cells.
The good news
As long as there are man-made water supplies Legionella presents an environmental health risk that needs to be avoided. There are physical steps that can be taken to help prevent Legionella contamination. These include reducing the risks of water stagnation, and ensuring the temperature of cold water is kept below 20°C and hot water is kept above 50°C (although not scalding). Better legislation, for example, monitoring the potential sources of Legionella infection such as in water towers, has lead to a reduction in the opportunities for infection. Secondly, employing guidelines to reduce bacterial viability using, among other steps, halogenation and monitoring can eliminate Legionella detection from man-made water systems. Surveillance and education programmes will both have their part to play in ensuring that outbreaks of Legionnaires’ disease are avoided in the future.
LAURA BOWATER
The Norwich Medical School, University of East Anglia, Norwich NR4 7TJ, UK
[email protected]
FURTHER READING
Fraser, D. (2005). The challenges were legion. Lancet Infect Dis 5, 237–241.
World Health Organization (2007). Legionella and the prevention of legionellosis.
Image: False-coloured scanning electron micrograph (SEM) of Legionella pneumophila bacteria. Eye of Science/Science Photo Library. False-coloured axial computed tomography (CT) scan of the lungs (blue/green) of a patient with Legionnaires' disease. The infection (yellow, lower right and left) is a sever form of pneumonia. ISM/Science Photo Library..
