Vaccines: the global challenge for microbiology
Issue: Why Microbiology Matters
05 May 2020 article
Vaccines are made from microbes that are dead or inactive, and these microbes stimulate an immune response to protect against disease. Not only do vaccines protect those inoculated, but they can also provide herd immunity. This section describes the crucial role vaccination plays in health.
The vital importance of vaccines
Norman K. Fry
As the media headlines increasingly remind us, the prevention, control and elimination of vaccine-preventable diseases (VPDs) do indeed present global challenges. Recent reports of outbreaks of measles in Samoa, the battle to regain polio-free status of several countries, and the enormous efforts to make one of the world’s deadliest diseases (Ebola) preventable and curable, all illustrate the vital importance of vaccines.
After clean water, vaccination is the most effective public health intervention in the world for saving lives and promoting good health. In 2019, The World Health Organization (WHO) re-affirmed its commitment to the prevention and control of communicable disease, including the VPDs. In the WHO’s list of ‘Ten threats to global health in 2019’, VPDs, vaccines and factors which can confound their successful delivery feature in most of them.
The success of vaccination over the last century in reducing death and disease caused by infectious diseases has been dramatic. Many viral and bacterial infections which historically disproportionally affected infants and children have been significantly reduced thanks to national immunisation programmes. However, we cannot afford to be complacent. Conflict leading to the breakdown of health service infrastructures and delivery systems, mass migration, movement of displaced persons (dramatically illustrated by the Rohingya refugee crisis in 2017), vaccine misinformation and hesitancy can all undermine the control of VPDs.
This issue contains articles on human papillomavirus (HPV) vaccination and herd immunity, and vaccine development and production. In the UK, the HPV vaccine has been offered to all girls in school Year 8 for over ten years and has continued to achieve very high vaccine coverage despite concerning declines in uptake in other European countries. From September 2019, the programme was expanded, with Year 8 boys now offered the vaccine alongside girls to help accelerate protection of both boys and girls from HPV-related cancers.
Innovative technologies are now playing an essential role in the development of new vaccines, and the hope for the future is that more diseases will become vaccine preventable across all age groups.
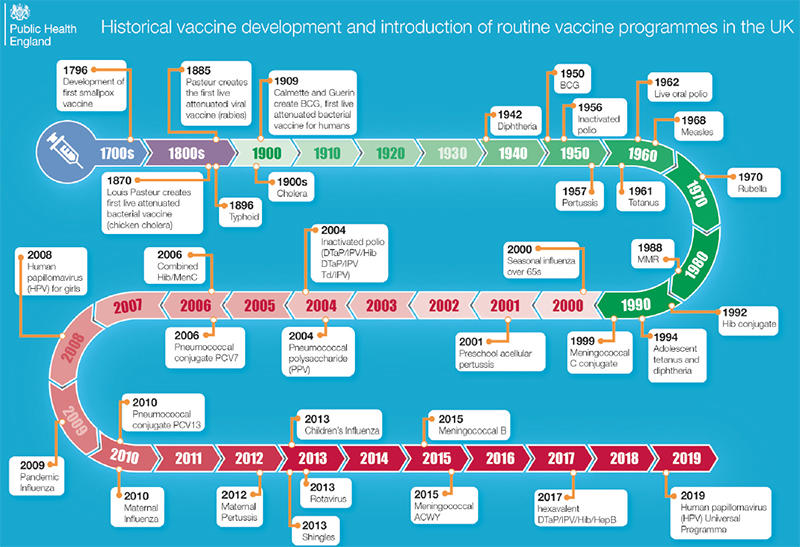
|
| Fig. 1. The figure shows the historical development of vaccines and the introduction of routine vaccine programmes in the UK. By kind permission of Public Health England. |
Further reading
Nature News. Make Ebola a thing of the past’: first vaccine against deadly virus approved; 2019. https://www.nature.com/articles/d41586-019-03490-8 [accessed 14 February 2020].
National Health Service. HPV vaccine overview; 2017. https://www.nhs.uk/conditions/vaccinations/hpv-human-papillomavirus-vaccine/ [accessed 14 February 2020].
Public Health England. Why vaccinate?; 2014. https://publichealthmatters.blog.gov.uk/2014/05/01/why-vaccinate/ [accessed 14 February 2020].
Wellcome Trust. This is the decade we made one of the world’s deadliest diseases preventable and curable; 2019. https://wellcome.ac.uk/news/decade-we-made-one-worlds-deadliest-diseases-preventable-and-curable [accessed 14 February 2020].
World Health Organization. Thirteenth general programme of work 2019−2023; 2018. https://www.who.int/about/what-we-do/thirteenth-general-programme-of-work-2019–2023 [accessed 14 February 2020].
World Health Organization. Ten threats to human health in 2019; 2019. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 [accessed 14 February 2020].

Norman K. Fry
Immunisation and Countermeasures Division, Public Health England – National Infection Service, London, UK
Norman Fry is a Consultant Clinical Scientist and Laboratory Surveillance Lead for Vaccine Preventable Bacteria in the Immunisation and Countermeasures Division, Public Health England – National Infection Service, London. He is Head of the Vaccine Preventable Bacteria Section which hosts the National Reference Laboratories for Streptococcus pneumoniae, Haemophilus influenzae, Bordetella pertussis and diphtheria. His laboratory also hosts the World Health Organization Collaborating Centre (WHO CC) for Streptococcus pneumoniae and Haemophilus influenzae (Heads: N.K. Fry and D. Litt) and the WHO CC for diphtheria and streptococcal infections (Head: Prof. A. Efstratiou). Norman is also Co-Editor-in-Chief for one of the Microbiology Society journals, the Journal of Medical Microbiology.
Why does microbiology matter?
Since this article was written, a novel coronavirus (later named SARS-CoV-2) was found to be responsible for a pneumonia outbreak that started in Wuhan City, Hubei Province, China. Due to the rapid spread of this virus the World Health Organization (WHO) declared a Public Health Emergency of International Concern and later made the assessment that the disease this virus causes, termed COVID-19, can be characterised as a pandemic.
If ever there was an answer to the question of why microbiology matters, this is certainly one. However, we should also not forget about the other infectious diseases, especially the ones for which we currently have vaccines for, and we should continue to maintain good vaccine uptake for those.
How are you striving to monitor the effectiveness of vaccination in the hope that it can control disease?
We work closely with our colleagues in Public Health England and the National Health Service (including scientists, epidemiologists and consultant medical microbiologists) to define outbreaks, describe the epidemiology of circulating strains and identify transmission pathways. We also collaborate with academia and other public health organisations, nationally and internationally, and are actively involved with several European laboratory and epidemiology networks. To monitor the effectiveness of vaccination nationally, it is essential to have both high-quality laboratory and epidemiological data. These data are also used to inform any potential changes to vaccine policy, following the approval and recommendation by the Joint Committee on Vaccination and Immunisation (JCVI), an independent departmental expert committee and statutory body which advises UK health departments on immunisation.
HPV vaccination and herd immunity
Kate Cuschieri and Heather Cubie
Papillomaviruses (PVs) are ancient and intriguing viruses. Biologists have been fascinated, challenged and perplexed by warts for centuries. In 1842, Dr Rigoni Stern, a Veronese physician, commented on the difference in cervical lesions in Catholic nuns compared to married women. Of course, at that time the transmissible element to cervical cancer was unknown, but the observation was astute. However, it was not until Harald Zur Hausen and his team discovered the fundamental links between certain human papillomavirus (HPV) types and cervical cancer, for which he later received the Nobel Prize, that this became more than a niche interest. Indeed, establishment of a viral aetiology to nearly all cervical cancers paved the way for one of the most influential, global public health interventions in modern history: HPV immunisation.
HPV vaccines: the building blocks
It has been known for several decades that virus proteins can self-assemble into virus-like particles (VLPs), either naturally or synthetically through expression, providing an invaluable delivery system for vaccines. VLPs are essentially empty shells which ‘look the part’ to the immune system. They produce a strong neutralising antibody response, considerably more potent than responses elicited by natural infection, but are incapable of replication. The discovery in 1990 by Jian Xhou and Ian Frazer in Brisbane that the two structural proteins of HPV could self-assemble into VLPs was a huge breakthrough and quickly led to investment in the development of HPV vaccines.
Initially, single valency vaccines containing only HPV 16 VLPs were trialled. These preceded the bivalent (Cervarix from GSK) and quadrivant (Gardasil 4 from SPMSD) vaccines which both confer protection against HPV 16 and 18, types associated with around 70% of cervical cancers. Gardasil 4 also provides protection against HPV 6 and 11 which cause around 90% of genital warts. Cross-protection has been demonstrated against non-vaccine HPV types, most notably by Cervarix for HPV 31, 33 and 45. More recently a nonavalent vaccine (Gardasil 9 from SPMSD) was licensed which confers protection against HPV 16, 18, 31, 33, 45, 52 and 58 as well as HPV 6/11 and is anticipated to directly protect against 90% of the types that cause cervical cancer. HPV 16 has a proven aetiology in non-cervical cancers, namely those of the vagina, vulva, penis, anus and oropharynx (Fig. 1), so clearly the vaccines have a reach for cancer protection beyond the cervix.
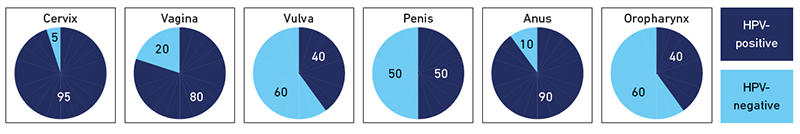
|
| Fig. 1. Attributable fraction of HPV in cervical, vaginal, vulval, penile, anal and oropharyngeal cancer. Cuschieri & Graham: taken from Molecular Microbiology – a guide to microbial infections 19th Edition, Eds Greenwood et al. Prevalences are based on Plummer et al. Lancet Glob Health 2016; 4: e609-16 and represent a global perspective |
Introduction and evolution of HPV vaccine within national programmes
Since 2006, 115 countries have introduced HPV immunisation as national pilots or full-scale programmes and the vaccine is now listed as an essential medicine by the WHO. Uptake has varied and depends on resource, competing healthcare priorities and perceptions of harm relative to benefits. Australia was the first country to offer a national HPV immunisation programme in 2007, with several countries following suit, including the UK in 2008. Initially, most programmes were female only, but increasingly males are also offered the vaccine. Gender-neutral vaccination has not been universally lauded, particularly where female uptake is high, and especially with global vaccine demand outstripping supply. Proponents contend that vaccinating boys accelerates and sustains herd immunity.
Furthermore, in addition to genital warts, males also shoulder a burden of HPV-associated cancer of the penis, anus and oropharynx. While these cancers are not common, they have a high morbidity and, importantly, are rising in incidence.
Data from the first randomised clinical trials (RCTs) of HPV vaccines were based on three-dose regimens and became the norm for implementation programmes. Now two-dose ‘prime-boost’ schedules are common, and, based on bridging studies that showed antibody levels after two doses were not inferior to those associated with clinical effectiveness, have been recommended by the WHO since 2014. More recently, analysis of the RCTs indicates that even a single dose may be protective against HPV infection, and prospective trials are underway to investigate this more comprehensively. Given the challenges of vaccination, particularly in low- and middle-income countries (LMIC) where 80% of the disease burden is manifest, this is a timely and exciting endeavour.
The effectiveness of HPV vaccines at the population level
The impact of HPV immunisation has been profound. In the UK, a 90% reduction of HPV type-specific infection (Fig. 2) in women who received the vaccine aged 14 was mirrored by an equivalent ~90% reduction in high-grade cervical lesions.
Impact is also being observed in the clinic, where the number of procedures required to remove cervical lesions has reduced over time in the vaccination era. We are now tantalisingly close to showing reduction, not just in disease but in invasive cancers.
Herd protection
Evidence of herd protection has accumulated for both quadrivalent and bivalent vaccines. One of the first key signals was reduction in genital warts in heterosexual males as a consequence of the then female-only vaccination programme in Australia. In Scotland, where vaccine uptake rates have been 80–90% since 2008, HPV vaccine-type infection and high-grade disease in girls aged 20, offered the HPV vaccine when aged 13–14 is the same, irrespective of immunisation status. As with any immunisation programme, high uptake should be encouraged to ensure maximal population-level benefits. Furthermore, men who have sex with men (MSM) are much less likely to gain herd protection from female-only programmes yet are at additional risk of HPV-associated disease compared to men who only have sex with women. Some countries have therefore introduced opportunistic programmes for MSM, including the UK in 2017, with monitoring systems in place to assess impact.
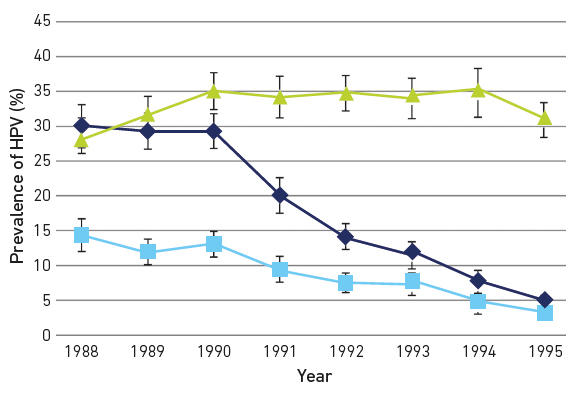
|
| Fig. 2. Type-specific prevalence of HPV in women attending for their first cervical smear in Scotland stratified by birth cohort showing reductions in vaccine-type infection and cross-protective types. Females born in 1988 and 1999 were largely unvaccinated; from 1991 the majority of females were vaccinated. Dark blue, HPV16/18; light blue, HPV 31/33/45; green, HR HPV other. Created by Kate Cuschieri from data available in Kavanagh et al. DOI:10.1016/S1473-3099(17)30468-1 |
New technologies to address global challenges
There is a global shortage of all HPV vaccines, with new production facilities under construction. Yet HPV vaccine remains one of the most expensive vaccines ever developed, and in some LMIC, especially in Africa and Asia, HPV types beyond HPV 16/18 contribute significantly to the cancer cases, suggesting not all vaccines will have equivalent efficacy in different countries. New technologies are required, and quickly, if the world is to respond to the WHO call for co-ordinated global action to eliminate cervical cancer [Dr Tedros Ghebreyesus, WHO Director General; 19 May 2018]. China and India have HPV vaccines under trial which should provide alternatives and bring down costs. Innovative collaborations between companies in developing countries and more established vaccine manufacturers will create further opportunities and competition.
Conclusion
As a result of immense efforts in producing, delivering and monitoring impact of HPV immunisation and herd immunity in high-income countries, global elimination of cervical cancer could be on the horizon, but it will require large-scale investment to provide high coverage of HPV vaccine to pre-sexually active girls and probably boys; political will and global action to address the United Nations Sustainable Development Goals (SDGs) of health for all and gender equality; and increased global investment in cervical screening for women over 30 to achieve high population coverage for at least the next two decades.
Further reading
Drolet M, Bénard É, Pérez N, Brisson M (on behalf of HPV Vaccination Impact Study Group). Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019;394:497–509. DOI:10.1016/S0140-6736(19)30298-3.
Kavanagh K, Pollock KG, Cuschieri K, Palmer T, Cameron RL et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017;17:1293–1302. DOI:10.1016/S1473-3099(17)30468-1.
Plummer M, de Martel C, Vignat J, Ferlay J, Bray F et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016;4:e609–616. DOI:10.1016/S2214-109X(16)30143-7.
de Sanjose S, Brotons M, LaMontagne DS, Bruni L. Human papillomavirus vaccine disease impact beyond expectations. Curr Opin Virol 2019;39:16–22. DOI:10.1016/j.coviro.2019.06.006.
Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985;314:111–114. DOI:10.1038/314111a0.

Catherine (Kate) Cuschieri
Scottish HPV Reference Laboratory, Department of Laboratory Medicine, 51 Little France Crescent, Edinburgh EH16 4SA, UK
Kate Cuschieri is a Consultant Clinical Scientist and current Director of the Scottish HPV Reference Laboratory. She is also lead for the HPV research group and Scottish HPV Archive, University of Edinburgh. She has been involved in monitoring the impact of the HPV vaccine on infection and associated disease in the UK.

Heather Cubie
Global Health Academy, University of Edinburgh, Edinburgh EH8 9AG, UK
Heather Cubie was a Consultant Clinical Scientist in Virology. She was Founder Director for the Scottish HPV Reference Laboratory and Scottish HPV Archive, retired in 2014 and is currently Senior Advisor to the Global Health Academy, University of Edinburgh. She also devotes much time to involvement in programmes of cervical cancer reduction in Malawi.
Why does microbiology matter?
Kate: From a clinical perspective the control of infection remains of extreme relevance for global health, including prevention through immunisation and public health interventions. Recent infectious outbreaks in the first part of the 21st century continue to emphasise this. Diverse microbiological expertise is needed to address the challenges imposed by infections, including basic scientists who can delineate mechanisms of pathogenicity, epidemiologists who can map infection and outbreaks – and health care scientists and clinical microbiologists who can support affected patients through laboratory testing and direct care.
What skills are required in your position on a day-to-day basis?
Kate: Currently, I am Director of the Scottish Human Papillomavirus (HPV) Reference laboratory, where we offer a national specialised service for testing of HPV. I am a consultant clinical scientist so in terms of qualifications I have a PhD, became state registered with the Health and Care Professions Council (HCPC) and then gained FRCPath by publication. As the laboratory acts as a hub for both testing and also advice on ‘things-HPV’, contemporary knowledge of the subject area is clearly required. In addition, I am lucky to be able to interact and collaborate with various multi-disciplinary groups that interface with HPV, including those involved in cervical screening, public health immunisation teams, clinical oncologists, quality assurance experts and academics/basic researchers. I also manage staff on the NHS and university side and support in the training and development of students and staff within and outside the microbiology sphere. Other required skills are the initiation and delivery of appropriate audit and research, the ability to contribute to national exercises that relate to the management of HPV infection and associated disease and finally (but importantly for a service laboratory!) the application of quality management systems to support clinical laboratory testing.
Vaccine development and production
Vanessa Terra and Brendan Wren
Vaccines have been one of humankind’s great successes in terms of combatting infectious diseases. However, despite their proven success, vaccines continue to be unavailable for most bacterial pathogens. Alarmingly, this includes the multi-antibiotic resistant ESKAPE group of pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species). Also, there is a pressing need for emergency response vaccines after natural disasters or civil strife (e.g. Vibrio cholerae) and for biothreat agents that could be released for nefarious purposes (e.g. Francisella tularensis, Bacillus anthracis and Burkholderia pseudomallei). Even if vaccines are available, such as glycoconjugate vaccines that prevent meningitis and pneumonia, they are often too expensive to be used in the low-resource settings where they are desperately needed.
Glycoconjugate vaccines for bacterial diseases
Current marketed vaccines include a number of different formulations that are effective in most high-income countries. These include component vaccines (tetanus and diphtheria), outer membrane vesicle-based vaccines used in the prevention of meningitis group B (e.g. Bexsero), live attenuated viral vaccines (such as the MMR vaccine) and glycoconjugate vaccines (Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenzae b). The latter are most desirable where glycans (e.g. O-antigens and capsules) are covalently linked to carrier proteins. They induce T-cell dependent responses that are long-lasting and are highly effective in infants and the elderly, with a remarkable safety record. Such vaccines are endorsed by the World Health Organization who recommend the development of glycoconjugate vaccines for more bacterial diseases. However, the use of the current carrier proteins (diphtheria and tetanus toxoids), means that new carrier proteins need to be developed to avoid cross-competition between different glycoconjugate vaccines.
Chemical conjugation
The development of new glycoconjugate vaccines continues at a pace. For example, TypBar-TCV and Zydus Cadila vaccines that protect against enteric fever caused by Salmonella typhi were licenced in 2013 and 2017, respectively. Both vaccines chemically conjugate the Vi capsule to tetanus toxoid and are effective in infants. Alternative strategies include the production of polysaccharides independent of the host pathogen including chemical and enzymatic synthesis of polysaccharides. Organic synthesis has been used to produce sufficient bacterial oligosaccharides for new vaccines against H. influenzae b (Quimi-Hi) and Shigella flexneri serotype 2a, which are currently in phase I clinical trials. Automated glycan assembly is another method used to generate polysaccharides independent of handling the pathogenic bacteria. This approach coupled to glycoarrays has been instrumental in the identification of epitopes from polysaccharide antigens that have been used to determine optimal oligosaccharide epitopes from serotypes excluded in current S. pneumoniae glycoconjugate vaccines. An alternative approach to producing a polysaccharide is by enzymatic assembly. Irrespective of how the polysaccharide is generated, all of these approaches require the need for chemical conjugation to the carrier protein. The disadvantages of the chemically synthesised glycoconjugate vaccines are that they are expensive to manufacture (due to multiple quality control steps), they don’t always fully cover strain/serotype variation and lack flexibility in the coupling of alternative carrier proteins to glycans.
Bioconjugation
An alternative to chemical conjugation is protein glycan coupling technology (PGCT) or bioconjugation that produces recombinant glycoconjugate vaccines in Escherichia coli cells that act as mini-factories for the single-step production of purified vaccines. The recombinant production of glycoconjugate vaccines in E. coli has many advantages including (i) no requirement to handle pathogenic bacteria, (ii) flexibility in the design of carrier protein/glycan combinations for tailor-made glycoconjugate vaccines and (iii) simplicity of the process means it is low-cost. Reduced production costs make this affordable for low-resource countries and the simplicity of the manufacturing process means that PGCT-derived vaccines can be produced within these countries, further driving down the cost of vaccines where demand is greatest.
Current PGCT-based vaccines in development include the refinement of S. pneumoniae vaccines with new carrier proteins from the host organism and further serotype coverage, new vaccines for F. tularensis, B. pseudomallei, Group A Streptococcus, K. pneumoniae, S. aureus, Shigella and E. coli. Some of these have been tested in phase 2 clinical trials and shown to be safe and effective. More recently, PGCT-derived vaccines have been developed for livestock (e.g. poultry, pigs and ruminants) where low-cost is paramount. Glycoconjugate vaccines have not previously been used in animals, so in an ironic twist, with over a billion glycoconjugate vaccines administered to humans annually, we have acted as the ‘guinea pigs’ for animals.
With the imminent post-antibiotic apocalypse, more than ever vaccine development is an obligation. In contrast to humans, vaccine development for animals has lagged. Appropriate vaccines for livestock have several advantages including disease prevention in animals (and humans for zoonotic pathogens), economic prosperity and a reduction in the use of antibiotics for animals. This means that the research, development and production of new human and animal vaccines is a timely pursuit and will continue to be a global imperative.

Brendan Wren
London School of Hygiene and Tropical Medicine (LSHTM), Keppel Street, London WC1E 7HT, UK
Brendan Wren gained a PhD in Biophysical Chemistry at the University of Leicester and published seminal papers on the effect of ionizing radiation on DNA. He then took a postdoctoral position in medical microbiology and has been at LSHTM for the past 20 years. He researches glycosylation in bacterial pathogens and developing a ‘glycotoolbox’ for glycoengineering; comparative phylogenomics and the evolution of bacterial virulence; and mechanisms of bacterial pathogenesis. This research has been used to develop glycoengineering to apply to the construction of affordable recombinant glycoconjugate vaccines.

Vanessa Terra
London School of Hygiene and Tropical Medicine (LSHTM), Keppel Street, London WC1E 7HT, UK
Vanessa Terra is a biochemist trained in Portugal. She obtained a PhD at the University of Leicester, working on pneumococcal interactions with mucin. Then she moved to the LSHTM and started working on vaccine development; more specifically protein glycan coupling technology (biological conjugation). Vanessa concentrates mostly on developing veterinary vaccines (PGCT), but also has an interest in the development of pneumococcal vaccines.
What advice would you give to someone starting out in this field?
Brendan: Follow what interests you and don’t be frightened of new research topics.
Vanessa: The most valuable advice I think I can give is to be passionate, be curious, be observant, be stubborn and don’t be afraid to explore – most important discoveries were made when we were looking for something else!
Why does microbiology matter?
Brendan and Vanessa: Microbes are the great survivors and occupy most niches on the planet, from deep-sea vents to hot springs. Understanding how and why they thrive is a true voyage of discovery.
Thumbnail image: didesign021/iStock.
Find out more about 'Vaccines: the global challenge for microbiology' in our digital content hub.
-
Vaccines: the global challenge for microbiology
Vaccines are made from microbes that are dead or inactive so that they are unable to cause disease. Not only do vaccines protect individuals, they can also provide herd immunity. We will explore four key areas of vaccination, including how vaccines work, are produced, more about herd immunity and eradicating disease.
Image: Nicola Stonehouse and Oluwapelumi Adeyemi.