A Gallium Trojan Horse?
Posted on January 11, 2024 by Dr Helen Chappell and Dr James Smith
Dr Helen Chappell and Dr James Smith take us behind the scenes of their latest publication 'Atomistic modelling and NMR studies reveal that gallium can target the ferric PQS uptake system in P. aeruginosa biofilms' published in Microbiology.
We (Dr Helen Chappell & Dr James Smith) head up research groups at the University of Leeds, UK in the School of Food Science and Nutrition. Our research is largely computational, using first principles Density Functional Theory (DFT) and Molecular Dynamics (MD) simulations, to answer questions about the chemistry of living systems. These computational modelling techniques are excellent for elucidating very specific bits of chemical behaviour, such as the complexation of metal ions to organic molecules and the thermodynamic feasibility of ion substitutions. In our recent paper in Microbiology, our groups, along with Dr Alex Heyam (Chemistry, Leeds), have used DFT and nuclear magnetic resonance (NMR) to look at the potential of gallium as a treatment for Pseudomonas aeruginosa infections in the cystic fibrosis (CF) lung and to see how it might function.
The mutations involved in the development of CF (principally ΔF508-CFTR) have been shown to impact iron metabolism, increasing levels of ferric iron in the CF lung. Unfortunately, this creates an environment where Pseudomonas aeruginosa thrives, with high levels of iron harnessed for the bacterium’s metabolism. This leads to infections that are incredibly difficult, and sometimes impossible, to treat. Normally this Gram-negative pathogenic bacterium deploys roving siderophores to chelate the free iron and deliver it to the bacterial membrane. However, it was noted in recent work, that P. aeruginosa variants that don’t produce siderophores, actually use the messenger molecule, Pseudomonas quinolone signal (PQS), to transport the iron to where it is needed for essential metabolism and proliferation. This is interesting, as it alludes to possible strategies for treatments designed to disrupt these iron-collection processes. This brings us to gallium.
Gallium has shown excellent potential as an antimicrobial agent in vitro, with low cytotoxicity in human lung fibroblasts. Phase 1 and 2 clinical trials have also been conducted, looking at the effectiveness of an intravenous gallium nitrate therapy as a strategy for interfering with iron metabolism in P. aeruginosa. What makes gallium such a useful potential therapeutic is that it is very similar in size and charge to ferric iron (Fe3+) and is easily (and mistakenly) scooped up by bacterial siderophores, which helpfully deliver the antimicrobial gallium to the bacterial membrane. However, we realised that no one had considered the potential for gallium to interfere with the PQS-Fe system of chelating iron. We wanted to know whether gallium would be stable in a Ga-PQS complex, and, more critically, whether it could displace iron from an already formed Fe-PQS system.
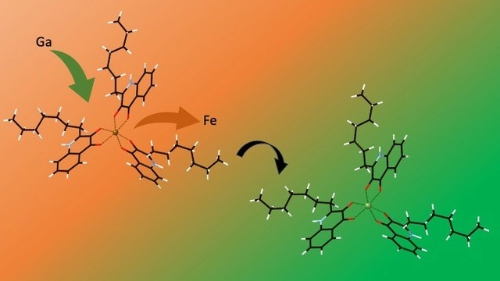
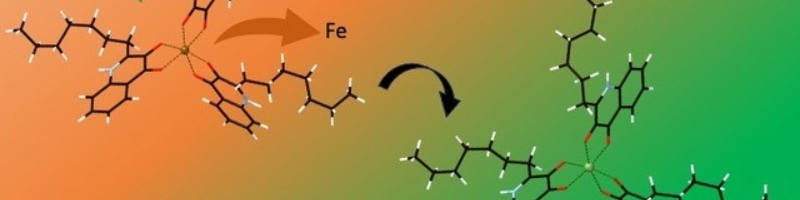
To do this, we carried out first principles DFT calculations, to assess the thermodynamic favourability (or you might prefer to think of it as ‘stability’) of the Fe-PQS and Ga-PQS complexes. What we discovered was that both ions are thermodynamically stable, but crucially, the displacement of Fe by Ga was favourable. In other words, we predicted, from first principles, that gallium could displace iron and therefore, bacteria that use PQS complexes to sequester iron from the environment, could be fooled into collecting gallium instead (Figure 1). When we submitted this result to Microbiology, the reviewers found it an interesting theoretical prediction, but they wanted to see if we could demonstrate it in solution. As we are essentially a computational-only laboratory, we sought help from friends in the School of Chemistry at the University of Leeds and came up with a set of NMR experiments to test our prediction. After a few false-starts (it turned that our solvent of choice needed to be methanol, not ethanol), we started to generate our NMR data. We had resonance signals for PQS on its own, PQS-Fe and PQS-Ga; luckily, the PQS-Fe and PQS-Ga complexes showed enough differences to clearly distinguish the two. So, the final experiment was to make up Fe-PQS and then mix in some gallium to see if the spectrum would shift from the Fe-PQS signal to the Ga-PQS signal. Unfortunately, we were not in the room when this experiment took place, but our PhD student, Isaac Noble, was there and he relayed the tremendous excitement of watching the signal clearly shift from Fe-PQS to Ga-PQS! These beautiful data can be seen in our recently published short communication. We hope that you agree that this is a very convincing result and that it bodes very well for the potential use of gallium as a ‘Trojan Horse’ therapy for treating iron-hungry Pseudomonas aeruginosa infections.
Our labs will continue working on the ‘molecules of infection’, with a particular interest in the extracellular polysaccharide matrix of biofilm-forming bacteria, and how small molecules (therapeutics say, or signalling molecules) do, or do not, navigate this sticky, hostile environment. This is an area that is sometimes overlooked in infection research, due to the experimental difficulties of examining and tracking the chemistry that plays out in this environment; fortunately, computational studies are very well placed to do the hard lifting on this one!
We would be very pleased to hear from anyone who is interested in our work, or the techniques we use: [email protected] (Dr Helen Chappell), [email protected] (Dr James Smith).
Thumbnail image: iStock/Wirestock