A Journey into Crassvirales phages and Bacteroides bacteria
Posted on September 27, 2023 by Bhavya Papudeshi
Bhavya Papudeshi takes us behind the scenes of their latest publication, 'Host interactions of novel Crassvirales species belonging to multiple families infecting bacterial host, Bacteroides cellulosilyticus WH2 published' in Microbial Genomics.
The story begins in the intricate world of the human gut, a bustling metropolis of microorganisms with trillions of bacteria going about their daily business. Among these inhabitants, the Bacteroides bacteria reign supreme as virtuoso recyclers, diligently breaking down complex polysaccharides that would otherwise remain undigested. Yet, much like any thriving city, the gut harbours dark alleys with bacteriophages –specific phage predators that prey on Bacteroides.
Enter the Crassvirales order, a group of phages with a remarkable history. Their story begins with the discovery of a phage named crAssphage by Prof. Robert A. Edwards( my supervisor and mentor at Flinders University) along with Prof. Bas E. Dutilh in 2014 (now professor at Friedrich Schiller University Jena, Germany). This phage gained fame as its genome appeared repeatedly in human microbiome samples and is present in most of us. Subsequent research revealed that these phages were not limited to one corner of the world; they could be found across continents, even in our primate cousins.
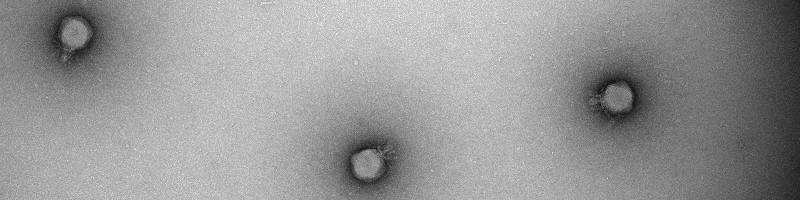
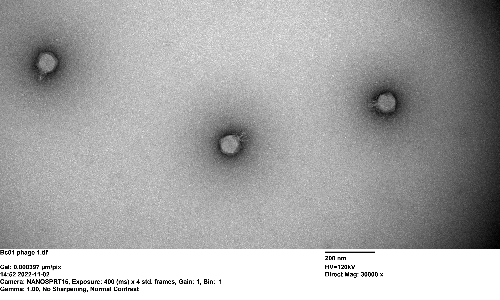
Fast forward to my research. We have isolated a unique set of 14 phages infecting Bacteroides cellulosilyticus WH2. These little warriors had genomes spanning a colossal 100,000 base pairs, reminiscent of the famous crAssphage. But these were not just clones, they belonged to three novel Crassvirales species;, Kehishuvirus sp. ‘tikkala’ strain Bc01, Kolpuevirus sp. ‘frurule’ strain Bc03, and ‘Rudgehvirus jaberico’ strain Bc11. What sets them apart from the four other isolated Crassvirales species is that although they span multiple genera and families, they infect the same bacterial host. It was like finding a hidden strategy used by these phages in the microbial war.
These phages were not locked in a co-evolutionary dance with their bacterial hosts. Instead, they seemed to be adapting to infect their hosts. This finding challenged our previous assumptions and opened doors to understanding the complex dynamics of phage-bacterium interactions. Our investigation zeroed in on a specific gene that encodes the tail spike protein, a crucial element for infecting their hosts. Despite belonging to different genera, the three novel Crassvirales species shared this crucial gene, with evolutionary pressure to stay the same suggesting its viral role in host interaction. Our analysis revealed that this tail spike protein binds to TonB-dependent receptors on the bacterial host's surface, initiating the phage's infiltration.
The implications of our research are profound, especially in the context of phage therapy. Our paper unveiled a story of adaptation, diversity, and a constant battle for survival in your gut. Specifically, a description of a strategy applied by these Crassvirales species to prey on Bacteroides species within the human gut. Understanding these mechanisms paves the way for more effective and sustainable phage therapy approaches by unravelling the intricacies of phage-host interactions and infection mechanisms. It's a world where science meets the promise of a healthier tomorrow, one bacteriophage at a time.
I would like to thank all the co-authors for their help in developing this story; it wouldn't have been possible without them. This work embodies the collaborative spirit that drives scientific progress, bridging multiple universities and countries, including Flinders University, University of Adelaide in Australia, San Diego State University, University of California Los Angeles, Washington University, and the University of Miami in the USA, as well as the University of Warsaw in Poland. This collective effort exemplifies how collaboration enriches and accelerates scientific discovery.