Beneath the surface: unravelling Mycobacterium abscessus morphotypes, lipid profiles and infection dynamics
As a PhD candidate at the University of British Columbia, I have had the opportunity to explore the complex world of Mycobacterium abscessus (MABS), a bacterial species notorious for its persistent infections and drug resistance. In collaboration with the Institut de Recherche en Infectiologie de Montpellier (IRIM) in France, our research team dedicated efforts to unravelling the intricacies of this pathogen, particularly its diverse morphotypes and their implications for infection dynamics. Our recent study, published in the Journal of Medical Microbiology, sheds light on the underlying pathogenic features of this globally concerning organism.
MABS poses a serious threat, particularly to immunocompromised individuals and those with pre-existing pulmonary conditions such as cystic fibrosis. Its high antibiotic resistance and ability to form persistent granulomatous abscesses in the lungs and other soft tissues make it a formidable challenge in clinical settings. For patients awaiting lung transplants, an active MABS infection can even preclude this life-saving procedure.
Early research has identified two distinct colony morphotypes in MABS: smooth (S) and rough (R). The S morphotype is characterized by uniform, glossy colonies, while the R morphotype exhibits irregular, textured colonies. These morphological differences are linked to the presence or absence of cell surface glycopeptidolipids (GPLs), respectively. Previous infection studies and clinical observations have revealed that the S morphotype is associated with early colonization, lower virulence and initial drug susceptibility. In contrast, the R morphotype is frequently isolated from persistent infections and demonstrates higher virulence and drug resistance. Despite the clinical significance of these morphotypes, the intricate relationships between GPL levels, colony morphology, and pathogenesis remain incompletely understood.
Our research aimed to bridge this knowledge gap by analysing strains isolated from the sputum of cystic fibrosis patients. A key observation in our study was that the majority of the clinical isolates exhibited both S and R colonies – a frequency much higher than previously reported. As we delved deeper, we realised that the traditional binary classification of 'smooth' and 'rough' failed to capture the full spectrum of morphological diversity we observed.
Through multiple rounds of subculturing, we unveiled a broad spectrum of 'intermediate' morphologies that fell between the S and R categories, with strain-specific variability. Some strains exhibited transient morphologies influenced by culture conditions, while others maintained stable phenotypes. This unexpected finding challenged the conventional understanding of MABS morphotypes and underscored the complexity of clinical isolates compared to laboratory strains.
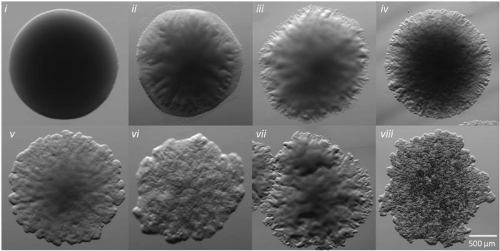
Perhaps our most intriguing discovery was a group of 'hybrid' strains that displayed R colony morphology but possessed GPL profiles more characteristic of S strains. This finding further refuted the prevailing premise that specific morphotypes are strictly associated with particular GPL profiles, revealing that the relationship between colony appearance and lipid composition in MABS is more intricate and variable than previously recognised.
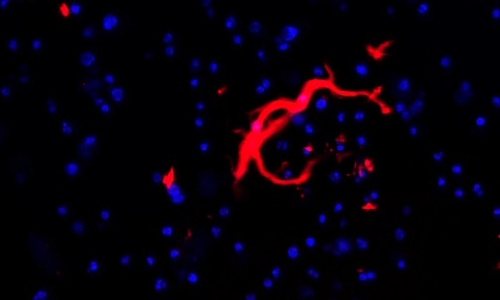
To better understand how these morphological features impact infection dynamics, we first employed advanced microscopy and thin-layer chromatography techniques to analyse colony morphology and GPL content and subsequently used a macrophage model to elucidate the relationship between morphotype, GPL profile, and infection behaviour. We strikingly found an inverse relationship between the clinical and laboratory morphotyped strains in their ability to infect and replicate in a population of macrophages. Most clinical R strains exhibited intracellular replication patterns more akin to S laboratory strains and overall demonstrated higher replication rates compared to their S counterparts. This observation is particularly significant given that the R morphotype is associated with persistent and aggressive infections. The rapid replication of R strains may contribute to their enhanced ability to overtake macrophages, escape, and subsequently form large extracellular cords that resist further immune clearance. This highlights the importance of studying clinical isolates, particularly since laboratory strains may not accurately reflect MABS behaviour in real-world infections.
Despite this broad inverse relationship between clinical and laboratory strains, we observed heterogeneity within both groups, confirming that morphotype is just one factor influencing infection trajectory. Our research has shed light on previously underestimated diversity among these pathogens and reshaped our understanding of morphotype and GPL roles in MABS infections. Given how central morphologies sit in MABS research, we believe a foundation must first be laid with a dedicated study to fully characterise the morphotypes microscopically and molecularly. This will bolster the existing pool of research and improve future work. By embracing a more comprehensive approach to morphotype classification, we can gain a better understanding of MABS infections and enhance our ability to combat them effectively. We hope that our findings will contribute to informing research strategies, improving diagnostic methodologies and the development of more effective therapeutic interventions.
The study of M. abscessus extends far beyond academic interest; it represents a critical endeavour to significantly impact the lives of those affected by this challenging pathogen. By disseminating our findings to a wider audience, we aim to catalyse further research and innovation in the field of microbiology, ultimately leading to improved outcomes for patients battling MABS infections.
