Can we evolve our own antimicrobials?
Posted on July 17, 2019 by Ayush Pathak
Our antimicrobials are slowly being rendered useless. Could it be that we have been producing outdated antimicrobials, while the organisms that naturally produce them have been continuously adapting and changing them? I am a master’s student at Imperial College London, and I have been researching this topic for the past nine months to try and find some answers.
Earlier this year, at the Early Career Microbiologists' Forum Summer Conference 2019, I presented a poster, ‘An antibiotic mediated evolutionary arms race between Alexander Fleming’s Penicillium rubens and a bacterium Bacillus muralis’. Considering my experimental findings, I plan to extend this project into my PhD.
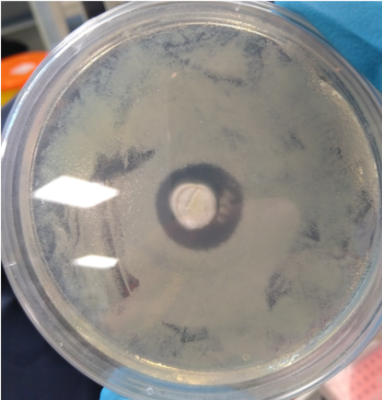
Penicilliums rubens (centre) co-cultured with the bacteria Bacillus muralis (yellow spots) surrounding it. Lack of growth of the bacteria in the periphery of the fungus indicates the production of penicillin by the fungus.
Antimicrobials are not artificially-made chemical compounds; they are naturally produced by various bacteria and fungi. Antimicrobials aid the antimicrobial-producing organisms in outcompeting the other groups of organisms that compete with the antimicrobial producers for resources. My research hypothesis is based on the idea that antimicrobial producers, using these compounds to outcompete other groups of organisms, face the same problem of development of antimicrobial resistance in their competitors. Thus, the antimicrobial-producing organisms might have developed certain countermeasures to deal with this problem; I would like to explore what these countermeasures are.
Over the past few decades, we have relied heavily on antimicrobials to fight bacterial and fungal infections. But the golden age of our antimicrobials is finally coming to an end. As more bacterial and fungal pathogens evolve resistance to antimicrobials, we are being forced to find novel solutions for dealing with bacterial and fungal infections. Thus, most of my research since my undergraduate dissertation has been aligned in the hope that I can contribute towards a solution to this crisis.
The inspiration for my current research project comes from the observations made by Sir Alexander Fleming himself. He observed that a contaminant fungus on his culture plates was killing the bacteria that had been cultured on these plates. He speculated that the fungus was producing a chemical compound that was killing the bacteria in its proximity on the culture plates. This compound was eventually purified, and he termed it as penicillin. The fungus which produced this compound has recently been re-identified as P. rubens. I decided to take this observation of his one step further. I simulated this antagonistic relationship between the strain of P. rubens discovered by Alexander Fleming and a bacterium B. muralis over evolutionary time. The bacteria were co-cultured with the fungus in petri dishes over a time period of two months. By doing so, an evolutionary arms race was simulated in which the fungus competed with bacteria for limited resources.
As the fungus produces penicillin, the bacteria will eventually develop resistance to penicillin, but then what does the fungus do? Are the fungi eventually outcompeted by the penicillin-resistant bacteria? Or can the fungus continually modify its own antimicrobials as the bacteria evolve resistance to each of the antimicrobials they produce? Or could it be that the fungus produces very small volumes of penicillin, such that the penicillin has very little inhibitory effect on the bacteria and does not induce penicillin resistance in the bacteria?
These are the questions I have been asking. I am particularly interested in knowing if the fungus can modify the penicillin compound it produces to cope with penicillin-resistant bacteria. If this hypothesis of continuous evolution of antimicrobials holds true, this could have far reaching implications in finding a solution to the crisis of antimicrobial resistance.
Until now, we have focused on finding new antimicrobials in nature or on artificially modifying already known antimicrobials. This has yielded limited success. With the approach summarised here, we could potentially evolve novel antimicrobials from various antimicrobial-producing organisms, in real time and in response to the development of specific kinds of antimicrobial resistance in pathogenic bacteria and fungi.

