Regulation of a carcinogenic genotoxin
The Roe Laboratory at the University of Glasgow, UK, has a diverse range of research interests, expanding out from the regulation and expression of virulence factors in enterohaemorrhagic Escherichia coli (EHEC) 0157:H7. As a postdoctoral research assistant in the Roe Lab, I work alongside PhD student Sofia Sandalli on the genotoxic secondary metabolite, colibactin, which is produced by biosynthetic machinery encoded within the polyketide synthase (pks) pathogenicity island carried by many B2-phylogroup E. coli.
Colibactin is a fascinating molecule for many reasons. It is most infamous as a procarcinogen, possessing two cyclopropane warheads that are capable of alkylating DNA and causing genomic damage and cytopathic effects that drive carcinogenesis. In particular, colibactin is aetiologically associated with colorectal cancer (CRC), and it is possible to identify a specific colibactin mutational signature in colorectal carcinomas. Further, pks+ E. coli are overrepresented in the colonic mucosa of patients with CRC in comparison to biopsies from healthy controls. With CRC incidence increasingly worldwide, and rates nearly doubling in younger people within the last decade, we need research to develop prophylactic treatments and novel therapeutics for treating this cancer. Inhibiting the production of colibactin by pks+ bacteria may present a possible therapeutic approach for preventing CRC incidences which arise from the genotoxin.
Characterising the factors that regulate the pks island is critical for understanding how to obstruct or repress colibactin synthesis. Sofia and I are particularly interested in how amino acid supplementation impacts colibactin expression, as the Roe Group has shown that certain amino acids downregulate the clb genes of the pks island which encode the apparatus for synthesising the genotoxin. Specifically, D-serine, the D-enantiomer of serine, shows promise in decreasing colibactin production and ameliorating DNA damage and cytopathic effects in vitro. Much of our research has been carried out in the probiotic E. coli strain Nissle 1917, which is utilised in treatment of numerous gastrointestinal disorders and is widely available to purchase over the counter. Given the prevalent use of Nissle 1917 for inflammatory intestinal issues, it is interesting that the strain also carries the pks island and is capable of producing functional colibactin. Sofia and I aim to characterise the exact molecular mechanisms by which D-serine and other amino acids are capable of repressing colibactin synthesis, ultimately being interested in whether amino acid supplementation lessens the development of pks+ bacteria-associated colorectal carcinomas in vivo.
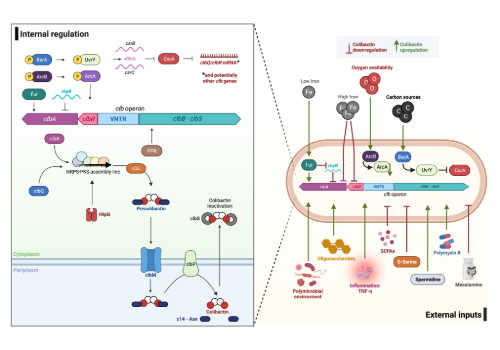
Our recent review sought to consolidate what is currently known about regulation of the pks island and colibactin synthesis, not only from a molecular perspective, but also considering the extensive extrinsic environmental factors that influence genotoxin production. Diet is an enormous modulator of the intestinal microbiome, and it is interesting to consider how amino acids, short chain fatty acids (SCFA), and iron bioavailability appear to interact with and downregulate the colibactin biosynthetic machinery, whilst conversely, inulin and galacto-oligosaccharides (GOS) enhance clb gene expression. Further, intestinal inflammation and hypoxia are associated with many gastrointestinal disorders and are present in CRCs. In our review we highlight the research that has shown how these conditions enhance colibactin production and drive tumorigenesis. The pks island is 54 kb, and is comprised of 19 genes which, as we discuss, appear to be regulated at multiple levels by many factors, with modulation by some of these factors appearing to occur on distinct or multiple genes in the colibactin biosynthetic locus. It was recently shown that ClbR is the main transcriptional activator of the clb genes, and as our understanding of how this protein interacts with other elements advances, it will hopefully provide a clearer picture of how colibactin synthesis is controlled.
Much is still unknown about the pks island and colibactin itself, which makes for a challenging and exciting area of research. We understand that the condition of the colonic environment hugely impacts colibactin production and genotoxicity, and that there is vast interplay between pks+ bacteria, the wider gut microbiome, and the host immune system. In the future, those of us working on colibactin will hopefully begin to unpick and unravel how external influences such as these alter the internal bacterial processes regulating production of the genotoxin. Sofia and I will be focused on deciphering amino acid induced colibactin repression by a combination of molecular, cellular, and in vivo based approaches, and we are excited to share more of this work in the future.
Thumbnail image: Image created with BioRender.com by Iris Floria
