Spotlight on Grants: Do tardigrades have a microbiome?
Posted on September 26, 2016 by Microbiology Society
Each year, the Microbiology Society awards a number of grants that enable undergraduates to work on microbiological research projects during the summer vacation. Over the next few weeks, we’ll be posting a series of articles from students who were awarded Harry Smith Vacation Studentships this summer. This week is Dana Barringham, a third year zoology student studying at Aberystwyth University.
FROM THE STUDENT: Dana Barringham
To the best of our knowledge, all animals, including humans, share their bodies with large communities of bacteria and other micro-organisms. Known as the microbiome, this community can provide essential additional functions for the animals, such as microbes in the guts of cows that enable them to digest cellulose.
But while the presence of a microbiome is well studied in larger animals, its existence in microscopic animals is less well understood. Is there a capacity for microscopic animals to contain a large microbiome, and if so, how does it affect them? With the help of my supervisor, Dr Arwyn Edwards, I decided to investigate this question by trying to determine the presence of a microbiome on one of my favourite animals, the tardigrade.
Tardigrades are tiny animals, averaging about 0.5mm in length. They are famous for their extreme survivability, with some species being able to withstand temperatures of almost absolute zero, and others surviving radiation more than 100 times the lethal dose for humans. There are even tardigrades that have survived in space, attached to the outside of a satellite.
However, there has been little work done on whether they have a permanent microbiome, and indeed whether a microbiome could potentially assist them in surviving these stressful conditions.
Before I could begin to look into their microbiome, I first needed to learn how to handle the tardigrades themselves. This has been, surprisingly, the hardest part of my project so far. I needed to isolate the tardigrades, without killing them, from the algae on which they feed. I have tried many possible combinations of centrifuging and sieving the tardigrades, but they are stubborn; they clung fiercely to the algae, no matter how long I centrifuged them. I then tried a different tack and individually picked out the tardigrades with a small glass micropipette. Even this method was a struggle, as oftentimes the algae would be sucked up along with the tardigrade, and too much could influence the results of the PCR.
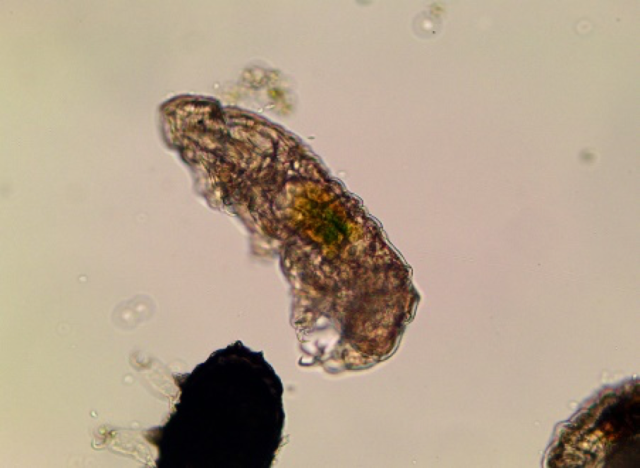
Dactylobiotus dispar under the microscope (Image courtesy of the researchers)
And so, now four weeks into my eight-week project, most of my time has been spent wrangling the tardigrades. I have done a few initial runs of PCR, using bacterial primers to try and identify any bacterial species that live in the tardigrades. The results from this have been promising so far, and my next step will be to sequence the DNA from the PCR products to determine the species of bacteria present in the sample. I will also be using fluorescence in situ hybridisation (FISH) to visualise any bacterial communities the tardigrades may contain, and this combined with electron microscopy will show where the potential microbiome would be located in the tardigrade’s body.
There is a chance, however, that this research could show that tardigrades do not have a microbiome at all. This presents an interesting question: given that almost all animals, even other microscopic ones, have a microbiome, why wouldn’t the tardigrade? Is it possible that the micro-organisms are unable to survive in the same conditions that the tardigrade can thrive?
My time working on this project so far has been an eye-opening experience. My supervisor has shown me what it is like to work in a real laboratory, and techniques I have learnt will aid me greatly in my tardigrade-related third year dissertation project, in which I will be studying their powerful heat shock proteins. The skills of running PCR and FISH, as well as using complex equipment, will be invaluable for my future career. In addition, learning how to design my own experiment and think of creative solutions to unexpected problems will be essential should I continue on to a PhD.
This has been an amazing opportunity from the Microbiology Society, and I will use this experience to help start my career in science.
FROM THE SUPERVISOR: Dr Arwyn Edwards
It has been a pleasure to welcome Dana to the lab, thanks to the support of the Microbiology Society. As the first “job” I had in microbial science was thanks to the Society’s vacation scholarship scheme in 2003, I recognise how important the experience can be in developing the career aspirations of undergraduate microbiologists.
Consequently, for me the most important aspect of the Harry Smith Vacation Studentship is the opportunity for students to experience life in a research lab, away from the structure provided by formal laboratory practicals. The scheme does this brilliantly by immersing the student in a real research environment and expecting them to take charge of their own project – often for the first time in their career.
The scheme also provides a rare opportunity for laboratories to explore: to test a new concept, or trying something ust for the sake of curiosity. For us, this has meant a foray into a new area for the lab, namely the study of tardigrades. My team regularly “meet” stray tardigrades when conducting microscopy for microbial enumeration in Arctic communities, but we had mainly considered them as “charismatic microfauna” to capture the imagination of students and the general public on the topic of extremophiles, rather than the focus of inquiry. Working with Dana at the intersection of our interests is hopefully paving the way for more detailed studies of tardigrade–microbe interactions – so long as our tardigrade subjects continue to cooperate!
To find out more about the Harry Smith Vacation Studentships, please contact [email protected]
