Validating ex vivo cornea for drug development in bacterial keratitis
My name is Dr Katarzyna Okurowska. During my time as a PhD student at the University of Sheffield, UK (in Dr Esther Karunakaran’s group at the Department of Chemical and Biological Engineering) and as a research technician on an MRC DPFS/Global Challenges project (led by Professor Peter Monk, Department of Infection and Immunity), I focused on establishing a reproducible and reliable ex vivo porcine cornea infection model to study keratitis. My goal was to help develop new antimicrobial treatments to prevent the progression of bacterial infection in a scenario reflective of in vivo infections, focussed mainly on Pseudomonas aeruginosa and Staphylococcus aureus.
P. aeruginosa keratitis in particular poses a significant challenge, especially in developing countries where immediate access to medical care may be limited. Pseudomonal infection advances rapidly, leading to stromal thinning and cloudy discolouration. In chronic stages, antimicrobial resistance, and complications, such as corneal lysis can result in vision loss or even loss of the eye. Thus, understanding infection’s progression and testing treatment efficacy on the ex vivo keratitis model could aid in the strategic selection of therapeutics with a higher likelihood of success in subsequent in vivo studies.
Reviewing existing literature revealed the absence of reliable ex vivo keratitis models for studying infection and testing antimicrobial treatments. Variations in experimental setups, corneal maintenance media, wounding techniques, strain types and inoculum sizes made comparing results or replicating experiments impossible. To address this, I streamlined the infection and drug application process to enhance predictability and reproducibility. I designed a glass mould to retain the cornea’s shape, secure it in place and allow the addition of bacteria and/or treatments to the corneal epithelium.
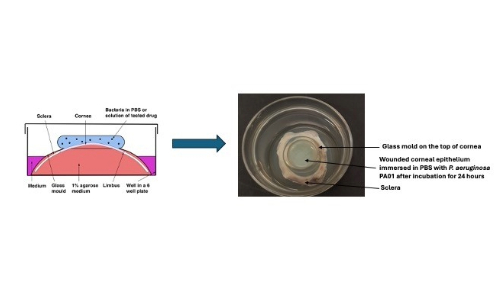
Our methodology in this article involved optimising the experimental protocol with previously published glass moulds and validating the model’s reproducibility. Importantly, the use of animal eyes sourced from abattoirs for this research not only reduced the need for sacrificing laboratory animals specifically for research purposes but also prevented the wasteful discarding of eyes that would otherwise occur in routine slaughter for human consumption. In this publication, I used two P. aeruginosa reference strains, PA14 and PA01, known for their biofilm formation capabilities and differing effects on host cells and clinical outcomes. We aimed to discern potential variations in their behaviour during different stages of infection, following treatment with antibiotics such as ciprofloxacin, gentamicin and meropenem.
An exciting discovery was that despite some limitations of this model, such as lack of host defences and tears, the response to antibiotic treatments aligned with trends found in the literature, suggesting similarities between our ex vivo keratitis model with other animal models in vivo and clinical studies on humans. Our ex vivo porcine keratitis model proved to be quick, economical, and highly reproducible.
The potential use of this model to assess therapeutics before in vivo testing is particularly exciting. It could reduce the need for sacrificing animals specifically for this research and provide an accessible alternative for many research groups. Developing new treatments often requires quickly validating compounds’ efficacy before proceeding to in vivo studies. Having an easily available ex vivo keratitis model would expedite the drug development and approval process.
Unlike in vivo corneal models, which face challenges in establishing early infection stages and can cause stress and pain to animals, our model allows studying both early and late infection stages. The quantity of retrieved bacteria at different time points is predictable. The use of a well-structured tissue also allows the study of biofilms, an important component in antimicrobial resistance.
Image: iStock/Inventori
