You can’t stop an outbreak without breaking a few eggs
Posted on February 15, 2017 by Anand Jagatia
Last year, a paper from Microbial Genomics described how scientists used molecular detective work to get to the bottom of an outbreak across Europe.
In June 2014, there was an outbreak of Salmonella at a hospital in Birmingham. Thirty-two people were affected, and one patient sadly died as a result of infection. Over the following weeks, more outbreaks began popping up in other parts of the UK. Several of these cases were linked to Chinese restaurants across England, and some were traced back to a kebab shop. In total, 247 cases of salmonellosis, caused by the bacterium Salmonella enteritidis, were reported by Public Health England (PHE).
The most likely explanation was that contaminated eggs or chicken were being served to customers who then got sick. But it was more than just a few random incidents – these events were part of a much larger outbreak across Europe, and hundreds of cases were also reported at the same time in Germany, France, Austria and Luxembourg.
So what was going on?
Dr Tim Dallman works at PHE’s reference laboratory for Salmonella infections. Whenever someone in England gets tested to see if they have a Salmonella infection, eventually their sample makes its way to the lab at PHE.
“Our job is basically to confirm it’s Salmonella, then compare their isolate with all the others that we’ve seen,” says Tim. “If several people have the same or very similar isolates, they might have had the same exposure – so it could be an outbreak that we need to investigate. This work helps us to protect the public from future outbreaks.”
Their aim was to find the source ultimately causing all these different outbreaks. What followed was a lot of scientific detective work, an international collaboration and a search across Europe for the bad eggs. They report their findings in Microbial Genomics.
*****
The investigation began by sequencing the DNA of all the isolates thought to be involved in the outbreak. By comparing sequences from different patient samples, the researchers could see how related the different isolates were, and reconstruct a genetic family tree, known as a ‘phylogeny’.
This particular tree was unusual because the Salmonella isolates that were implicated in the outbreak were split into three distinct branches, or ‘clades’, with each branch representing a cluster of strains that are more closely related to each other than the rest. The team estimated that these three clades branched off in around 2012.
“We were thinking, why are we seeing these strains that have diverged four years ago, all at the same time?” Tim says. “That seemed counterintuitive to us.”
PHE were also working with the Food Standards Agency to interview people and find out where they might have picked up their Salmonella infection from. This information was used to build a ‘traceback network’, which showed the locations where people became infected, where the eggs were distributed from, and who supplied them. Once the team had this information, the penny dropped.
“It struck us how the food traceback network was similarly complex to the phylogeny,” says Tim. “We wondered whether there was any relationship between the two, whether one was telling us information about the other.”
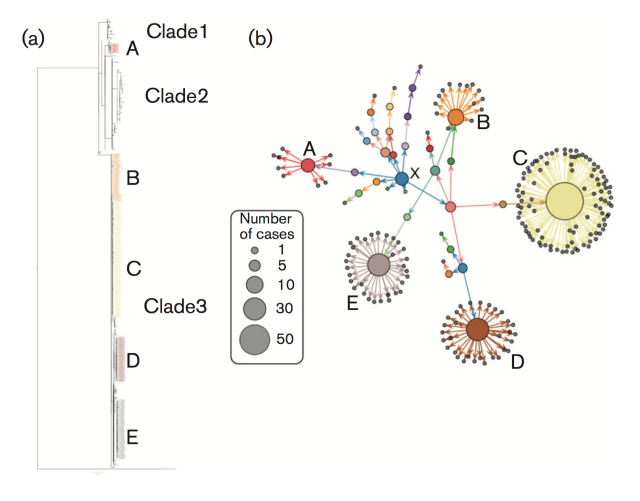
(a) Phylogeny based on genetic sequences of the Salmonella isolates
(b) Traceback network, showing how different outbreaks in the UK were connected
Collaborating with Dr Thibaut Jombart at Imperial College, the team was able to demonstrate that these two ‘maps’ were statistically related to each other. They showed that, if two cases were close to each other on the traceback network, they were also close to each other on the phylogenetic tree. So, people who picked up their infections from similar egg suppliers were infected by more closely related strains.
While this was going on, researchers in Europe were performing similar analyses in their own countries. In the course of their investigations, authorities in France were able to track down an individual egg that was positive for one of the Salmonella strains involved in the outbreak. Using the labels found on all eggs in the EU, they traced it back to an egg distributor in Bavaria.
This company had three different production facilities in south-west Germany, and one across the border in the Czech Republic. The French team showed that the strain from this egg fell onto a particular clade of the phylogenetic tree, and that it came from one particular site from the Bavarian producer.
“This was enough to enable German authorities to do further sampling, and they found Salmonella bacteria at a second site. And all of those isolates clustered into one distinct clade of outbreak cases,” says Tim.
As other countries involved in the outbreak began to do further tracebacks, it became clear that all of the cases from Clade 1 of the phylogenetic tree came from one of the Bayern Ei sites, and all the cases from Clade 2 came from a second.
This explained why the strains were related, but clustered into distinct groups. The infected chickens all came from an original stock, but were kept at separate sites to lay the eggs. No Salmonella strains from Clade 3 were found at these sites, although some cases from this group were linked to the company via traceback.
“What was exciting about the paper for us is that with whole genome sequencing, we could see the intimate relationship between the outbreak strains,” says Tim. “ It told us the story of how they were being moved around this company and then across Europe.
“Using sequencing is very strong forensic evidence, so authorities take it seriously and are more likely to follow it up with action,” says Tim. “Previously, the investigation may have just resulted in looking to prosecute the Chinese restaurants for food hygiene practices, but that’s not a great public health intervention in terms of targeting the actual problem at source.”
“With whole genome sequencing surveillance, we’re beginning to find that a lot of these outbreaks aren’t just located in a small part of England, they’re often international. Whole genome sequencing enables us to get a much better understanding of the true epidemiology – where strains are circulating, how they’re evolving – and ultimately it enables us to make more targeted interventions.”
