Chikungunya: a decade of global pain
Issue: Zoonotic diseases
05 November 2015 article

It is has been 10 years since chikungunya emerged from the forests of Africa and started its global journey, from Africa out across the islands of the Indian Ocean to India and Southeast Asia, China and the Americas. The virus first emerged to prominence early in 2005 on the French island of La Réunion.
La Réunion, an island off Madagascar in the Indian Ocean, is part of France and has a population of around 750,000. In 2005, a third of the population became infected with chikungunya virus and there were 237 deaths (approximately 1 per 1,000 cases). Healthcare workers and scientists were rushed to La Réunion from France to deal with the outbreak.
There are three major strains of the virus: East Central South African (ECSA), West African and Asian. These differ in their geographical distribution, transmission by mosquitoes and genome sequence. Chikungunya virus is classified as an alphavirus within the family Togaviridae. These are small, enveloped positive-sense RNA viruses. Related viruses include Semliki Forest virus, Sindbis virus, Ross River virus and Venezuelan equine encephalitis virus. Chikungunya, Sindbis and Ross River viruses are often grouped as ‘Old World alphaviruses’ since, until recently, they were not present in the Americas. The Old World alphaviruses generally cause arthralgia (joint pain). The ‘New World alphaviruses’ found in the Americas include Eastern, Western and Venezuelan equine encephalitis viruses. These are more dangerous; they cause encephalitis (brain inflammation) and can be fatal.
Transmission
Chikungunya virus is transmitted by mosquitoes of the Aedes genus, specifically Aedes aegypti and Aedes albopictus. These two mosquitoes are now widely spread around the world. A. albopictus, the Asian tiger mosquito, was disseminated from South-east Asia through international trade in houseplants and tyres in which mosquito larvae survive in pools of water. These two mosquitoes transmit both chikungunya and dengue and, as they prefer to bite humans, they are very effective disease vectors and are responsible for most cases of chikungunya. Generally, at least for the ECSA strain, A. aegypti is the vector in urban areas and A. albopictus is the vector in suburban and rural areas. In the 2005 epidemic in La Réunion, a point mutation in the E1 virus envelope glycoprotein of an ECSA virus increased the ability of this virus to replicate in A. albopictus. This almost certainly contributed to the massive outbreak on La Réunion. The Asian strain of the virus, currently circulating in the Americas, predominantly in the Caribbean, is transmitted preferentially by A. aegypti. Differences in the genome between ECSA and the Asian strain seem to be preventing the same point mutation in the E1 glycoprotein adapting the Asian strain to transmission by A. albopictus.
Chikungunya derives its name from an outbreak of the disease in Tanzania in the 1950s. The name derives from the local language Makonde and translates as ‘that which bends up’. It refers to the fact that people with chikunguyna, due to pain in their joints and muscles, have a stooping, bent posture. The majority of infections are symptomatic and the incubation period is 2 to 7 days. Chikungunya causes an abrupt onset severe fever and debilitating joint and muscle pains, which are generally asymmetric and affect the ankles, wrists, fingers, knees, shoulders and elbows. Frequently there is also a rash. Headache, fatigue, nausea, conjunctivitis, and in newborns, meningitis and encephalitis, have also been described. The acute disease generally lasts for a couple of weeks. The differential diagnosis is dengue fever. Indeed, it is likely that some of the epidemics of fever, described since colonial times throughout the tropics as dengue fever, were caused not by what we now term dengue virus but by chikungunya virus.
Structure and pathogenesis
We know relatively little about the molecular virology of chikungunya. In contrast, other alphaviruses have been much studied for many decades, particularly Semliki Forest virus, a close relative of chikungunya virus, Sindbis virus and Venezuelan equine encephalitis virus. The alphaviruses have relatively few proteins. A polyprotein is processed into four functional proteins, which together act as the virus replicase replicating the virus RNA and transcribing a subgenomic RNA. The subgenomic RNA provides an amplification mechanism for producing large numbers of viral structural proteins, a capsid protein and two or three virus envelope glycoproteins. The envelope glycoproteins mediate entry into cells and are determinants of host range and transmission. As with many, perhaps all, RNA viruses, in order to replicate in mammalian cells, the virus must suppress the interferon response. For the alphaviruses, non-structural protein 2 (nsP2) is responsible for this. The alphavirus capsid and nsP3 proteins also have important roles to play in modulating the functions of mammalian cells. For the mosquito vectors, as for all invertebrates and plants, the key suppressor of virus replication is not interferon but RNA interference (RNAi). Arboviruses, at least most of them, do not, or do not strongly, antagonise the RNAi response resulting in a low-level persistent infection with minimal fitness cost to this virus distribution system.
We also know relatively little about the pathogenesis of the human disease. There is a transient high titre viraemia (virus in the blood), which peaks 1 to 2 days post-infection and can last for a week. This drives acute inflammatory and immune responses with most commonly high levels of interferon-alpha and interleukins-1, -6, -12 and -18. The virus is observed in monocytes in the blood and in fibroblasts and macrophages in tissues. Interestingly, macrophages are infected by the uptake of apoptotic debris from virus-infected cells, not by purified virus. Whereas the acute infection, as characterised by fever, generally resolves within 2 weeks, the polyarthralgia generally lasts longer; one study estimated a mean duration of 90 days. For some people there is chronic disease with long-term arthralgia and in some cases arthritis. The immunological findings in these patients are different to those of rheumatoid arthritis with no C-reactive protein, no rheumatoid factor, a different cytokine profile in the blood and joints and no evidence of autoimmunity. Anti-chikungunya virus IGM antibodies can persist for years, indicative perhaps of a persistent infection. Consistent with this, in one case, virus RNA and proteins were detected in synovial macrophages 18 months post-infection. We know that in mouse models alphaviruses can persist in tissues for long periods of time. It would not be surprising if chikungunya virus can do so in a tissue such as the joint. Studying this is not easy for ethical reasons and most people with joint pain are sensitive to having their joints biopsied. Close cooperation between virologists and surgeons undertaking elective surgery in locations which have had many cases of chikungunya might further inform the pathogenesis of the chronic disease. The chronic joint disease in chikungunya raises the interesting question; could viruses be responsible for a subset of arthritic diseases worldwide?
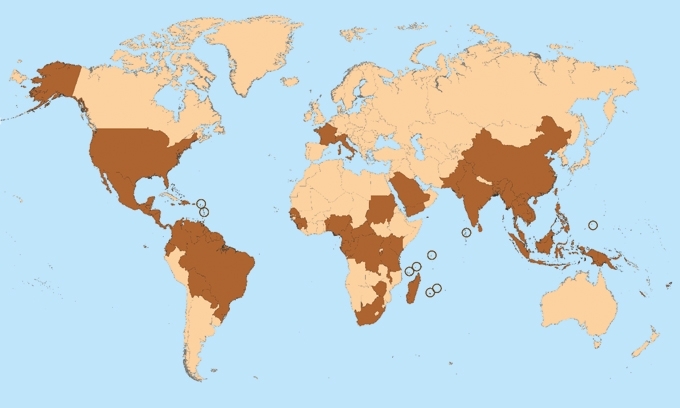
THE DARK BROWN DENOTES COUNTRIES AND TERRITORIES WHERE CHIKUNGUNYA CASES HAVE BEEN REPORTED (AS OF 10 MARCH 2015). DOES NOT INCLUDE COUNTRIES OR TERRITORIES WHERE ONLY IMPORTED CASES HAVE BEEN DOCUMENTED.
As with many infectious diseases, an understanding of the pathogenesis is greatly facilitated by the study of animal model systems. Mice are not easily infected with chikungunya virus but inoculation close to a joint results in local joint swelling. Infection of mice with poor type-I interferon responses has been studied as a more disseminated model of infection. Macaque monkeys experimentally infected with chikungunya provide a better model of the human disease. As with humans, acute disease is characterised by a high-level transient viraemia with virus in monocytes, rash, severe fever and joint swelling with persistence of virus RNA and proteins in lymphoid tissues and liver.
The outlook
At present there are no licenced vaccines for chikungunya though several candidates have been shown to be efficacious in pre-clinical testing including in macaques. There are vaccines based on attenuated chikungunya virus and other alphaviruses, virus-like particle systems, RNA and DNA constructs, and other engineered virus vector systems including vaccinia (MVA) virus and measles virus. At least three candidate vaccines have been through or are in Phase I human clinical trials. It should be relatively easy to protect against the acute disease since we know for most alphaviruses that good antibody responses correlate with protection. More difficult factors might be longevity of immunity, distribution in the tropics and cost per dose. Until the virus hit the Americas, developing a vaccine for this disease was generally not considered to be commercially viable.
In December 2013, the virus reached the Caribbean. Since then there have been an estimated 1.5 million cases in the Americas, mostly in the Caribbean but also in the countries in South America. As elsewhere, this has been an explosive epidemic in a naïve population. It will probably resolve with time and the virus will disappear back into the forests of Africa and Southern Asia, where it presumably persists in an arboreal cycle between monkeys and mosquitoes; whether the monkeys and mosquitoes of South America are also able to sustain this virus remains to be seen.
So far chikungunya cases have been recorded in 60 countries with, according to some estimates, over five million cases. Europe hasn’t escaped the pain. There was an outbreak in Italy in 2007. A traveller from India introduced the virus into the Po river delta where there are plenty of mosquitoes. This resulted in local transmission and almost three hundred cases. Vector control by the local public health authorities brought the outbreak under control. In 2010, there were autochthonous cases in southern France. Aedes albopictus is now established around the northern shores of the Mediterranean and it is estimated by the European Centre for Disease Prevention and Control that within a few decades the range of this vector could extend across northern Europe into southern Britain.
JOHN FAZAKERLEY
Director, The Pirbright Institute, Pirbright GU24 0NF, UK
[email protected]
Image: Coloured transmission electron micrograph of chikungunya virus particles. CDC/Cynthia Goldsmith, James A. Comer and Barbara Johnson/Science Photo Library. Map of countries and territories where chikungunya cases have been reported; it is updated weekly if there are new countries or territories that report local chikungunya virus transmission. Taken from Centers for Disease Control and Prevention/National Center for Emerging and Zoonotic Infectious Diseases (NCEZID)/Division of Vector-Borne Diseases (DVBD)..
