Campylobacter: breaking the spiral of infection
Issue: Zoonotic diseases
05 November 2015 article
Campylobacter jejuni is an excellent example of a zoonotic pathogen, but for many years it was arguably the least known of the common bacterial food pathogens.
Those of us who grew up in the UK in the late 1980s may remember the injustice of no longer being allowed to eat uncooked cake mix, due to the risk of Salmonella infection. More recently, many will recall headlines about the 2011 German Escherichia coli outbreak. Campylobacter very rarely makes the national headlines, although the number of Campylobacter cases have remained relatively steady over the last few decades, with an estimated annual incidence of ~280,000 UK cases.
In 2012, the BBC Face the Facts programme asked the public to name bacteria that cause foodborne illness. Not surprisingly, many people mentioned Salmonella and E. coli, some people even named Listeria, but Campylobacter was the great unknown. However, that picture is changing rapidly and in the past year the public profile of Campylobacter has been raised significantly, mainly due to the Food Standards Agency (FSA) publishing data on the percentage of Campylobacter-positive meat samples from retail sources. While insiders were not surprised by the levels of Campylobacter reported (64–79% of chicken meat was positive), the numbers were widely publicised, putting pressure on the poultry sector and retailers to address the problem.
Why is tackling Campylobacter so important?
For such a common pathogen, it may seem surprising that there is so little public awareness. There may be several reasons for this: firstly, Campylobacter infection is typically a self-limiting illness. Symptoms usually last between 3 and 10 days and include fever, stomach cramps and diarrhoea, but rarely vomiting. Although infection can lead to potentially severe autoimmune complications, these are rare and illness is typically self-resolving, requiring little professional attention. This means that the infection burden is economic rather than medical, costing an estimated £900 million each year in the UK, an amount the recovering economy could put to much better use! Secondly, Campylobacter is ever-present and hard to eradicate. Food safety measures implemented to reduce Salmonella, another zoonotic pathogen linked to poultry, have had little effect on Campylobacter levels. Birds show few symptoms when infected, so poultry producers have no outward signs of infection. Additionally, as a result of the rapid slaughter and processing methods, uninfected birds may become contaminated during processing.
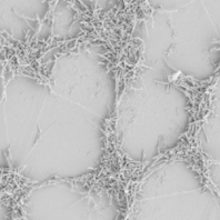
SCANNING ELECTRON MICROGRAPH (SEM) IMAGE OF A CAMPYLOBACTER JEJUNI BIOFILM FORMING ON A SOILED SURFACE. THE CAMPYLOBACTER IS ABLE TO ATTACH TO THE SOILING PARTICULATES MORE EASILY THAN IT CAN TO A CLEAN SURFACE, AND SO THE BIOFILM CAN GROW MUCH QUICKER.
The Campylobacter conundrum
In addition to the Campylobacter retail survey, the FSA have been active with several public awareness campaigns, focusing on how people should handle raw chicken. The food industry has argued against the policy of ‘naming and shaming’, and to an extent we sympathise. Campylobacter has had many millions of years to learn its trade, and they are now expected to produce immediate results. Campylobacter is a fastidious organism, requiring a rich growth medium, a narrow range of temperatures and modified atmospheric conditions for growth. Researchers working with the bacterium have many anecdotes of how Campylobacter cultures left on the bench for a few hours die and how opening an incubator door one too many times led to the loss of a weeks’ worth of experiments. Yet, despite this fastidious nature, Campylobacter is able to survive for extended periods within the food chain (a contradiction, which, within the field is nicknamed the ‘Campylobacter conundrum’). We still don’t fully understand how this survival is achieved, or how to interfere with these mechanisms. This is not good news for poultry producers who serve the consumer demand for low-cost chicken, but want to avoid possible future government sanctions for non-compliance. The ACT (Acting on Campylobacter Together, #actonfarm on Twitter) campaign shows the drive of regulators and industry to solve this problem.
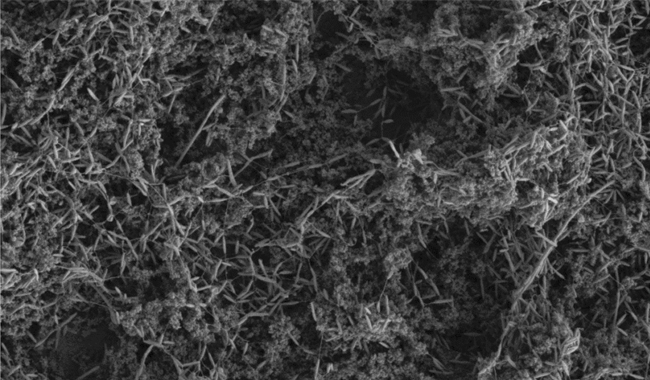
A C. JEJUNI BIOFILM HAS FORMED AROUND A SMALL WOODEN SPLINTER. THIS SPLINTER COULD NOT BE SEEN BY EYE.
Where can we stop Campylobacter?
Campylobacter is commonly found in the intestine of birds and animals, and also survives in agricultural environments. Flocks commonly become Campylobacter-positive in the latter stage of their growth, and infection spreads rapidly within flocks. In the kitchen the bacteria either cross-contaminate other foodstuffs and equipment (one reason why you shouldn’t wash your chicken!), or survive when the meat is improperly cooked. There are several points in the process where interventions may be successful. Firstly, preventing birds from becoming infected. This is currently attempted by strict biosecurity measures, but the FSA results show these measure are unsuccessful in their current form. Other interventions being trialled include feed additives and improved disinfection procedures. Secondly, contamination of meat may be prevented at the abattoir stage, and we discuss some ideas on how Campylobacter survives in that environment in the diagram above. Promising interventions include surface chilling, disinfectant rinses and combinations of steam and ultrasound. Finally, consumers can protect themselves with good kitchen hygiene and food preparation. Retailers are doing their bit, for instance with the ‘double-bagged whole chicken’, where consumers don’t touch the chicken until it’s fully cooked.
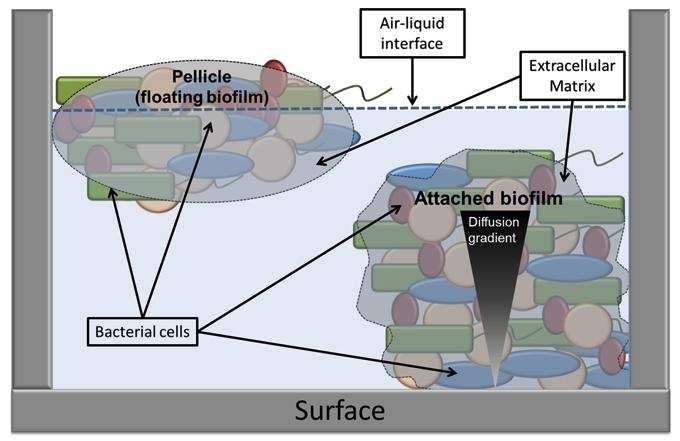
SCHEMATIC REPRESENTATION OF TWO DIFFERENT TYPES OF BIOFILM AND KEY COMPONENTS.
A potential Campylobacter survival strategy
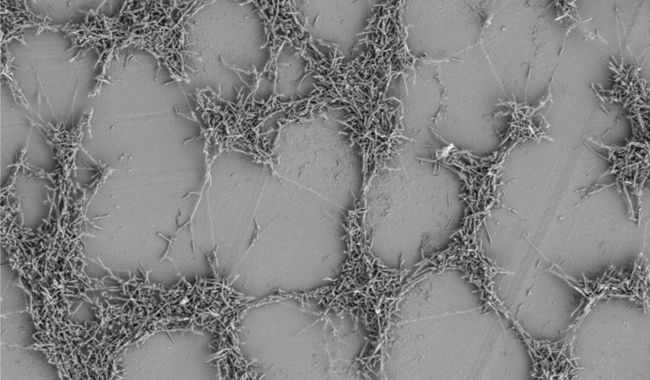
Biofilms, formed of bacterial communities enveloped by an extracellular matrix, are an important mechanism for bacterial survival of suboptimal conditions. Typically, biofilms contain multiple bacterial species and can be dense enough to see with the naked eye. Reduced oxygen levels can be found in the deeper levels of biofilms, perfect for survival of microaerobic bacteria such as Campylobacter. Similarly, multispecies biofilms produce a wide range of metabolites, allowing many different bacteria to thrive by co-operatively producing the nutrients required by the community. There is now a large body of evidence indicating that Campylobacter is able to not only integrate into existing multi-species biofilms, but can form de novo biofilms on food chain relevant surfaces such as stainless steel. Surface soiling with meat juices or other organic matter increases Campylobacter’s ability to attach to surfaces, increasing biofilm formation. We, and others, have shown that DNA is an important component of the extracellular matrix, and its enzymic degradation allows rapid dispersal of attached Campylobacter populations. Motility also appears to be an important factor in biofilm formation, allowing bacterial cells to migrate towards, and away from, surfaces and helping to form surface attachments in the initial stages of biofilm formation.
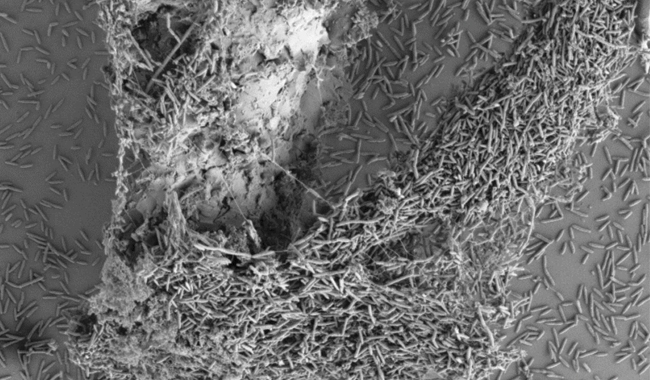
A C. JEJUNI BIOFILM FORMING ON A PLASTIC SURFACE.
The future of biofilm investigation
The majority of bacteria exist in mixed species biofilms, and we expect Campylobacter to behave no differently. Multi-species biofilms are, by their very nature, extremely complex, and this complexity has, until recently, hindered their study. Advances in ‘omics technologies, in silico modelling and the development of techniques which allow single cell interactions to be studied are allowing us to better understand how multi-species biofilm communities are able to thrive. These new technologies should increase our understanding of Campylobacter and how it is transiting the food chain, and may lead to the development of knowledge-led Campylobacter eradication strategies throughout the food chain.
What should we do until then?
It should be stressed though that the consumer plays a central role in breaking the infection cycle and is firmly in control of their health. They must be aware of the risk posed by zoonotic pathogens such as Campylobacter. Consumers should be cautious when handling raw chicken and wash their hands and utensils immediately afterwards in order to ensure that cross contamination does not occur. Campylobacter does not tolerate freezing very well and so freezing chicken before consumption will reduce the number of viable bacteria on the meat. Washing raw chicken should be avoided, as contaminated water could be splashed onto surfaces or other foods. Fortunately, Campylobacter is also relatively easy to destroy by cooking, so ensuring that chicken is cooked adequately before consumption should be enough to stop infection. Following these simple guidelines should be adequate to ensure that you stay Campylobacter-free!
HELEN BROWN
Cardiff School of Health Sciences, Llandaff Campus, Cardiff Metropolitan University, Western Avenue, Cardiff CF5 2YB, UK
[email protected]
ARNOUD VAN VLIET
Institute of Food Research, Colney Lane, Norwich NR4 7UA, UK
[email protected]
FURTHER READING
Acting on Campylobacter Together. Food Standards Agency. http://microb.io/ 1JVwg0U. Last accessed 25 August 2015.
Brown, H. L. & others (2014). Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Applied Environmental Microbiology 80, 7053–7060.
Campylobacter. Food Standards Agency. http://microb.io/1PwuwJA. Last accessed 25 August 2015.
Okshevsky, M. Regina, V. R. & Meyer, R. L. (2015). Extracellular DNA as a target for biofilm control. Current Opinion in Biotechnology 33, 73–80.
Teh, A. H. T., Lee, S. M. & Dykes, G. A. (2014). Does Campylobacter jejuni form biofilms in food-related environments? Applied Environmental Microbiology 80, 5154–5160.
Image: SEM image of C. jejuni biofilm on a plastic surface. Dr Louise Salt, IFR. SEM image of C. jejuni biofilm on a soiled surface. Dr Louise Salt, IFR. SEM image of C. jejuni biofilm on a wooden splinter. Dr Louise Salt, IFR. Schematic representation of two types of biofilm and key components. Helen Brown and Arnoud van Vliet..
