Burkholderia bacteria: natural alternatives to synthetic pesticides
05 November 2019

Micro-organisms have fascinating lifestyles and many of those that live in the natural environment carry out beneficial or detrimental functions depending on the situation in which they find themselves. Gram-negative Burkholderia represent one group of bacteria with exactly these ‘Dr Jekyll and Mr Hyde’ tendencies.
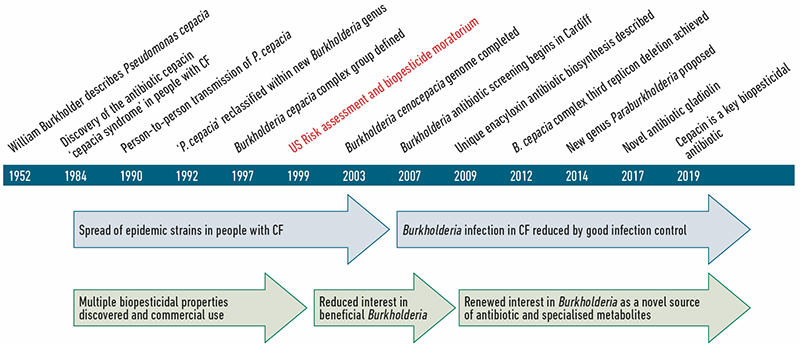
The major negative effect Burkholderia are known for is infection within a range of hosts including humans, animals and plants; in contrast, they also mediate highly beneficial interactions. A timeline of these positive and negative traits, together with key landmarks in our understanding of Burkholderia are shown (Fig. 1). Here we expand on how the dilemma of inherent Burkholderia pathogenicity initially led to a decline in the exploitation of these bacteria as beneficial biopesticides in the 1990s. However, renewed interest in these bacteria as a source of natural products and antibiotics, coupled with a greater understanding of their diversity and genomics, has provided an opportunity to repurpose Burkholderia as safe biopesticides (Fig. 1).
Burkholderia bacteria are diverse!
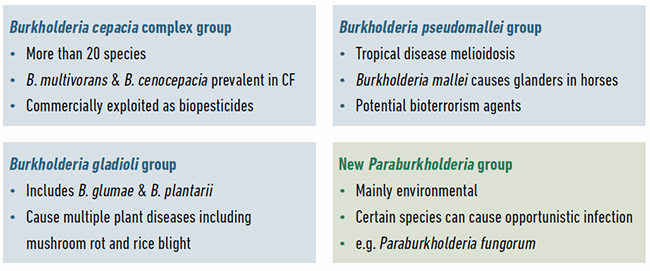
The evolutionary history of Burkholderia bacteria continues to be unravelled with the ongoing discovery of novel species and regular updates to the taxonomic distinction between genera. From their initial discovery in 1952 as the cause of onion rot, Burkholderia bacteria were associated with pathogenicity. The majority of research on these bacteria has focused on understanding the species most associated with disease (Fig. 2): the Burkholderia pseudomallei group as primary pathogens; the Burkholderia cepacia complex as opportunistic pathogens well known for causing lung infections in people with cystic fibrosis (CF); and the Burkholderia gladioli group of plant pathogens. However, all Burkholderia are capable of living freely or as symbionts in the natural environment, and are not always found associated with infection.
Recent taxonomic analysis has split Burkholderia further, defining the novel genus Paraburkholderia (Fig. 2). Paraburkholderia species are rarely encountered as pathogens and hence some researchers have referred to them as the environmental or beneficial group. But this is an oversimplification as certain Paraburkholderia species can cause opportunistic human infections, nearly all Burkholderia and Paraburkholderia occur widely in the natural environment, and finally, multiple beneficial interactions have been characterised for both groups.
|
| Fig. 1. Key events in our understanding of Burkholderia bacteria as both pathogens and beneficial bacteria. A basic timeline from the discovery of these bacteria to current research on how they work as biopesticides is shown. |
Burkholderia produce multiple specialised metabolites
The ability to antagonise and kill a range of other micro-organisms was a key feature that drove the initial exploitation of Burkholderia bacteria as biopesticides. Although a number of antibiotics such as cepacin (Fig. 1) were historically known to be produced by Burkholderia, recent genomic analysis has shown that they encode multiple biosynthetic gene clusters for the production of various specialised metabolites. Burkholderia can produce polyketide antibiotics (e.g. enacyloxin and gladiolin; Fig. 1) that are made by large, modular biosynthetic gene clusters. They can also make both ribosomally synthesised and post-translationally modified peptides (e.g. capastruin) and non-ribosomally synthesised peptides and lipopeptide antibiotics (e.g. icosalides). The use of genome mining to identify novel biosynthetic gene clusters combined with sensitive analytical chemistry greatly enhances specialised metabolite discovery in Burkholderia bacteria.
Large unusual genomes and a non-essential third replicon
Given their functional diversity, it is not surprising that Burkholderia also have very large genomes to encode these multiple capabilities. Their genomes are nearly twice the size of most bacteria, ranging from six to nine million base-pairs of DNA. Burkholderia also represent a minority of bacteria that package their genomes into multiple large replicons instead of having just one chromosomal piece of DNA. The majority of Burkholderia and Paraburkholderia species have two-replicon genome structures. The Burkholderia cepacia complex group of species (Fig. 2) have a three-replicon genome. In 2012 (Fig. 1), it was found that the Burkholderia cepacia complex third replicon was not an essential piece of DNA and could be genetically removed. Third replicon mutants lost a number of functions, including the production of antifungals and, most strikingly, their virulence in a range of infection models.
|
| Fig. 2. Example species and the properties of different groups of Burkholderia bacteria. The four groups of Burkholderia currently being studied by researchers and example species within them are shown. |
Burkholderia biopesticides
Multiple chemically synthesised pesticides are used to protect crops against a range of pathogens and during vulnerable stages of their growth. Germinating crop plants are highly prone to attack by pathogens, with damping-off disease (the failure of seeds to germinate or rapid wilting of recently emerged seedlings) caused by a range of fungi and fungal-like organisms called oomycetes. During the 1990s, several Burkholderia biological control products were registered with the US Environmental Protection Agency (EPA) and used successfully in a range of applications, including protection against damping-off disease.
Products, including Deny® and Blue Circle® manufactured by Stine Microbial Products, were described as containing [Burkholderia cepacia] strains M54 and J82. These strains and multiple other isolates with biocontrol properties were subsequently identified as the species Burkholderia ambifaria. In parallel with this beneficial use as biopesticides, [Burkholderia cepacia] bacteria were also emerging as devastating CF pathogens (Fig. 1). In 1999, after assessing the risks of Burkholderia biopesticides, a moratorium was placed on the registration of all new biopesticides until they could be proven safe (Fig. 1).
Cepacin is a key biopesticidal Burkholderia metabolite
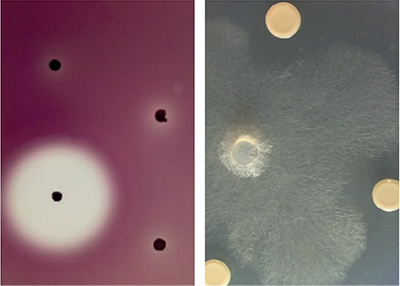
In 2015 we began a genome mining study to find out which specialised metabolite biosynthetic gene clusters were encoded and expressed by biopesticidal Burkholderia ambifaria strains. We found that Burkholderia ambifaria strains encoded at least 38 different specialised metabolite biosynthesis gene clusters and had a broad range of antimicrobial activity (Fig. 3). We also identified a novel gene cluster controlled by the bacterial signalling system quorum sensing that, when mutated, stopped Burkholderia ambifaria from producing the historical antibiotic cepacin.
|
| Fig. 3. The antimicrobial activity of Burkholderia ambifaria. Antagonism against the Gram-negative Pectobacterium is shown as a zone of clearing around a black, antibiotic-producing Burkholderia colony (left) and inhibition of the filmentous growth of the oomycete damping-off pathogen Pythium by 3 Burkholderia colonies (right). |
The Burkholderia ambifaria biopesticidal strains had been shown to protect crop plants such as peas against damping-off disease caused by Pythium. Simply coating pea seeds with the Burkholderia ambifaria enabled protection when they were planted in pathogen infested soil (Fig. 4). When the same biocontrol assay was carried out with the Burkholderia ambifaria cepacin mutant protection was lost. We also generated a Burkholderia ambifaria third replicon mutant which, despite the loss of nearly 1 million bases of DNA, still had a functional cepacin biosynthesis gene cluster. The third replicon mutant still gave excellent biocontrol protection of peas but crucially when tested in a mammalian infection model it had lost its pathogenicity and ability to persist.
Future perspectives – what is needed to allow safe use of Burkholderia as biopesticides?

|
| Fig. 4. Biopesticidal protection of peas by Burkholderia ambifaria in soil infested with Pythium. Pea seeds were planted in soil infested with the damping-off pathogen Pythium and as a result failed to germinate (left), coated with Burkholderia and germinated successfully when planted in the same infested soil due to protection from antimicrobial cepacin production (centre), and planted in un-infested soil where they germinated successfully (right). |
It is now clear that Burkholderia represent a novel source of antibiotics and encode a huge diversity of specialised metabolite biosynthetic gene clusters. To fully harness the potential of Burkholderia as natural biopesticides, we have to find out exactly how they work, for example as we have done by uniquely linking cepacin production to protection against Pythium damping-off disease. We also must find out what happens to biocontrol strains when they are applied to crops and left in the field environment. To be environmentally safe they need to decline naturally, and not build up or interfere with other microbial systems that maintain soil health.
Most importantly we need to make sure any Burkholderia biopesticides will be safe for use and will not cause infection. This can be done via an attenuation strategy; for example, removing the third replicon greatly reduced Burkholderia ambifaria pathogenicity, and now with modern strategies to develop safe bacterial vaccines, we can also consider removing other infection-mediating genes from biocontrol strains. Commercial confidence in the potential of Burkholderia biopesticides also appears to be re-emerging in the US, with Marrone Bio Innovations® marketing heat-killed Burkholderia products possessing nematocidal and mitocidal properties. Overall, producing enough food to feed the growing human population is a major global challenge, and this needs to be balanced against the environmental impacts of intensive agriculture. Burkholderia and other natural microbial biopesticides, which will not impact the environment, have huge potential to help sustain future agricultural production.
Acknowledgements
Alex J. Mullins was funded by a Biotechnology and Biological Sciences Research Council (BBSRC) South West doctoral training partnership (BB/M009122/1). Alex J. Mullins and Eshwar Mahenthiralingam acknowledge current funding of their biopesticide research by the BBSRC (award BB/S007652/1). We are also grateful to Professor Greg L. Challis and his research group (University of Warwick, Coventry, UK), for their continuing collaboration to understand the chemistry and biosynthesis of Burkholderia natural products.
Further reading
Mullins AJ, Murray JAH, Bull MJ, et al. Genome mining identifies cepacin as a plant-protective metabolite of the biopesticidal bacterium Burkholderia ambifaria. Nat Microbiol 2019;4:996– 1005.

Alex J. Mullins
Microbiomes, Microbes and Informatics Group, Organisms and Environment Division, School of Biosciences, Cardiff University, Cardiff CF10 3AX, UK
Alex J. Mullins graduated from Cardiff in 2015 with a BSc in microbiology, having written his dissertation on Burkholderia bacteria in the Mahenthiralingam Lab. He decided to pursue this research further and started a PhD, investigating the genomic and antibiotic properties of the historical biopesticide Burkholderia ambifaria. Following his PhD, he continues to research Burkholderia as a postdoctoral researcher.

Eshwar Mahenthiralingam
Microbiomes, Microbes and Informatics Group, Organisms and Environment Division, School of Biosciences, Cardiff University, Cardiff CF10 3AX, UK
After completing a degree in Applied Biology at Cardiff and a PhD with the Medical Research Council, Eshwar Mahenthiralingam carried out 10 years of postdoctoral research on cystic fibrosis at the University of British Columbia, Canada. He joined Cardiff University in 1999. In addition to teaching microbiology and guiding his research group, he is also Co-Director of Research and works on the school’s management team.
What inspired you to become a microbiologist?
Alex: I became interested in microbiology at school when we learnt about major historical diseases such as bubonic plaque and the role of microbes in vital nutrient cycles such as nitrogen fixation. I saw so much potential in these organisms that you could not see and, inspired by a biology teacher with a degree in microbiology, I followed up by studying and now researching microbiology at university.
What skills are required in your position on a day to day basis?
Eshwar: When I was training as a microbiologist, the key skill areas I developed were in molecular biology and genomic analyses, and these, combined with bioinformatic knowledge, are now crucial for many areas of microbiology. Other key research skills include perseverance, curiosity, and the confidence to take risks and occasionally do ‘whacky’ experiments, many of which layed the foundations for successful programmes such as our current Burkholderia antibiotic discovery research.
Images: Fig. 1. Eshwar Mahenthiralingam and Alex Mullins.
Fig. 2. Eshwar Mahenthiralingam and Alex Mullins.
Fig. 3. Alex Mullins.
Fig. 4. and thumbnail. Alex Mullins.
