Schoolzone: Bacteriophage practical
18 February 2013
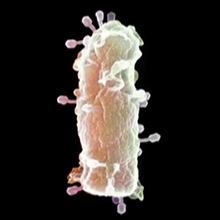
Aspects of virology feature in many A-level specifications but practical virology is virtually absent in school because of difficulties in handling the micro-organisms (both perceived and real). Bacteriophages (viruses that only infect bacteria) are relatively easy to handle and can be used to illustrate many concepts of virology, such as counting infectious virus particles.
A plaque assay is a technique for detecting viruses. The underlying principle offered here is a serial dilution (gradually diluting a suspension of bacterial viruses and testing for infectivity). The outcome should be an agar plate covered with ‘plaques’ (holes in a field of constant bacterial growth), where the virus has infected and killed the host bacterium.
The school-friendly method detailed here offers a step-by-step guide to carrying out a plaque assay using T4 bacteriophage and its host, Escherichia coli.
Aim
To calculate the number of viable phage in a suspension.
Method
Note: Aseptic technique should be used throughout this experiment
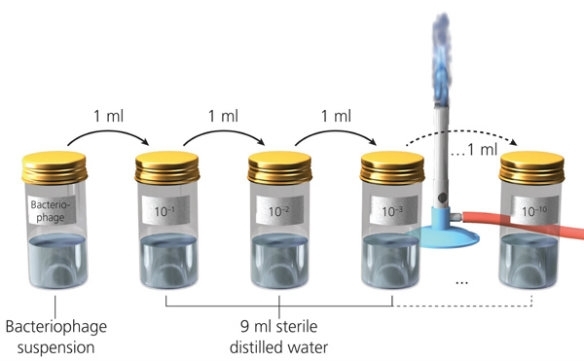
Stage 1 – Preparing your dilution series of T4 bacteriophage
Each group of students requires 10 nutrient agar plates, 10 bottles each containing 9 ml sterile distilled water and 10 sterile pipettes.
- Label one agar plate ‘10–1‘ and the next one ‘10–2‘ and so on until ‘10–10‘.
- Label the sterile water bottles ‘10–1‘, ‘10–2‘ and so on until ‘10–10‘.
- Transfer 1 ml of bacteriophage suspension into the sterile water bottle labelled ‘10–1‘ and mix well.
- Using a sterile pipette (not the one used previously), transfer 1 ml out of the ‘10–1‘ bottle and place it into the ‘10–2‘ bottle. Replace the lid and mix well.
- Continue this process (transferring 1 ml from one dilution to the next using a new sterile pipette each time) until you have made the ‘10–10‘ dilution.
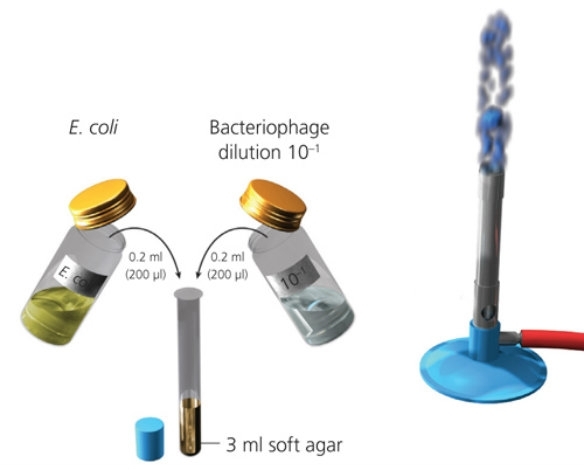
Stage 2 – Inoculating cultures of bacteria with T4 bacteriophage dilutions
The following steps (1–6) must be completed for each dilution
- Collect your ‘10–1‘ agar plate and the bacteriophage dilution bottle labelled ‘10–1‘.
- Collect 3 ml of molten soft agar from a water bath (set at 50°C). When opening the soft agar, ensure you are working aseptically (near a Bunsen burner).
- Using a sterile pipette, transfer 0.2 ml (200 µl) of E. coli culture into the soft agar bottle/test tube.
- Using a sterile pipette, transfer 0.2 ml (200 µl) of your phage dilution into the soft agar bottle/test tube.
- Place lid on bottle/test tube. Carefully rotate/roll the bottle between two fingers in an upright position, to mix the solution (do not shake).
- Carefully pour the inoculated soft agar onto your ‘10–1‘ agar plate. Replace the lid of the agar plate and carefully rotate the plate in a circular motion so the soft agar is evenly spread over the underlying agar. Leave the plate for approximately 10 minutes for the soft agar to set (at room temperature).

Once all agar plates have been inoculated with soft agar and the agar has set, invert the plates and incubate them at 30°C for 24 hours. Empty bottles/test tubes should be sterilised and cleaned before reuse.
Results
The bacteria grow as a lawn (a field of confluent growth) across the agar. Small circular gaps in the lawn of bacteria are known as plaques. Here, bacteria have been killed by the bacteriophage so are unable to grow.
Count the number of plaques at a dilution where there are between 20 and 200 plaques present. This will allow calculation of number of plaque-forming units (PFUs) per ml of original suspension using the formula:
PFU/ml= (number of PFUs)
(dilution x 0.2)
- Enter the number of PFUs into the top line of the equation.
- Enter the dilution you chose for your average PFU where the equation says ‘dilution’. For example, if you chose 10–3, enter 0.001, or if you chose 10–7, enter 0.0000001.
Notes
– Soft agar is half-strength nutrient agar. It must be sterilised and be molten (kept at 50°C) for students to use.
– The bacteriophage must be able to infect the bacterium you use. They are highly specific.
– Advice on bacteriophage and obtaining a culture can be sought from culture suppliers/collections
JAMES REDFERN, Manchester Metropolitan University
