Behind the research of ‘A novel variant of the Listeria monocytogenes type VII secretion system EssC component is associated with an Rhs toxin’
Posted on June 7, 2023 by Kieran Bowran
Kieran Bowran takes us behind the scenes of his latest publication 'A novel variant of the Listeria monocytogenes type VII secretion system EssC component is associated with an Rhs toxin’ published in Microbial Genomics.
My name is Kieran Bowran, I’m a PhD student at Newcastle University, UK.
My current research area is the substrates of the type VII secretion system (T7SS) in Gram-positive organisms, investigating their genetic diversity and their predicted biochemical activity against competitor bacteria. Gram-positive organisms like Streptococcus and Bacillus use their T7SS to target competitor bacteria using a diverse range of anti-bacterial toxins. Bacteria can also accumulate immunity genes which encode protective proteins against the toxic activity of the secreted substrates. Further understanding the activity of the T7SS and how bacteria utilise it across a range of environments will further knowledge of bacteria-bacteria interactions and investigating the cellular targets of their antibacterial toxins may highlight new potential antibiotic targets in the future.
Recently we collaborated with Arnoud van Vliet (University of Surrey) who had compiled an extensive dataset of Listeria monocytogenes genomes. This dataset allowed us to investigate our previous findings, published in Microbiology, on a much larger scale and truly assess the genomic diversity of the T7SS and its substrates in L. monocytogenes. As part of the analysis, we identified a novel type of predicted T7SS toxin – a rearrangement hotspot (Rhs) domain protein in a subset of strains (Figure 1). Previously, RHS toxins have been implicated in the type VI secretion system of Gram-negative organisms like Pseudomonas, Salmonella and Vibrio species which utilise them as both anti-bacterial and anti-eukaryotic toxins [1,2].
Having access to such a large dataset made it possible to observe T7SS genetic trends across the whole species, as all evolutionary lineages and nearly all clonal complexes were represented. Our previous analysis was carried out on a smaller sample size, and it was encouraging to see the results of that analysis be mirrored in a larger, more representative dataset. We were able to observe that specific T7SS variants dominated certain lineages and clonal complexes of L. monocytogenes, something which previously we were unable to do.
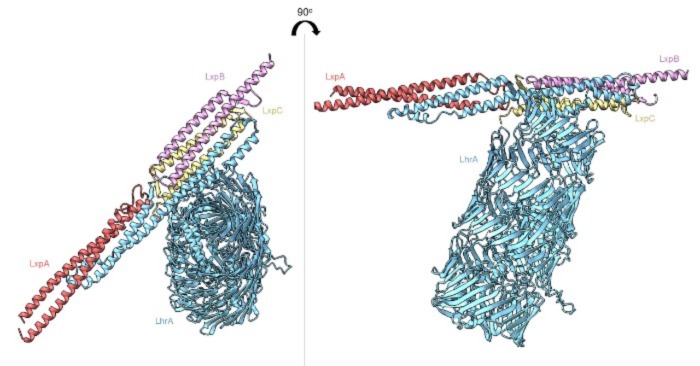
Large datasets can be unwieldy simply due to the large volumes of data and information available; however, collaborating alongside Arnoud allowed us to access the relevant information to answer our questions. It’s always exciting identifying something novel in science, and in the case of the Rhs protein it was really satisfying seeing the AlphaFold predicted model and the symmetry in the β-barrel cocoon that surrounds the predicted toxin domain.
One of the underlying questions research groups are trying to answer is how these substrates are being exported by the T7SS across the cell envelope. Commonalities in protein structures and associated partner proteins are now being identified across multiple bacterial groups. The more information there is on the diversity of T7SS substrates will hopefully allow these questions to be answered. The Rhs protein is much larger than other known T7SS substrates and poses interesting questions on how it would be exported by the secretion system complex.
A large number of predicted T7SS substrates have unknown C-terminal toxin domains. Further research on these proteins may highlight interesting biochemical activity which may open future routes for antibiotic targets against the critical priority groups of multidrug-resistant pathogens.
References:
[1] Gϋnther, P., Quentin, D., Ahmad, S., Sachar, K., Gatsogiannis, C., and Whitney, J. C. (2022). Structure of a bacterial Rhs effector exported by the type VI secretion system. PLoS Pathogens. 18(1):e1010182. DOI: 10.1371/journal.ppat.1010182.
[2] Jurėnas, D., Rey, M., Byrne, D., Chamot-Rooke, J., Terradot, L., and Cascales, E. (2022). Salmonella antibacterial Rhs polymorphic toxin inhibits translation through ADP-ribosylation of EF-Tu P-loop. Nucleic Acids Research. 50(22), pp13114-13127. DOI: 10.1093/nar/gkac1162.

