Could antibiotics be making Campylobacter jejuni more dangerous?
Posted on March 4, 2019 by Matthew Whelan
The January Junior Awards for Microbiology (JAM) talks took place in Birmingham on the 25 January. PhD student Matthew Whelan was speaking, and in the second of our blogs from speakers at the JAM talks, Matthew takes us through his research into Campylobacter jejuni.
I’m very lucky to carry out my research in the world of host–pathogen interactions, as I find it amazing to be able to see how entirely different biological systems interact with each other. My PhD focuses on investigating the global regulation of virulence phenotypes of Campylobacter jejuni.
C. jejuni is the leading cause of bacterial food-borne pathogenesis globally. Over 550 million cases of Campylobacteriosis are reported yearly. What’s really interesting about C. jejuni is it exists primarily as a commensal (where the bacteria benefit but the host is unaffected) in birds, particularly broiler chickens. However, upon transmission into the human small intestine, which usually takes place through ingestion of undercooked chicken, the C. jejuni become pathogenic and invade the body through the wall of the small intestine, causing severe gastroenteritis. In roughly one in 1000 cases, the infection can lead to very serious immune disorders such as reactive arthritis and Guillain-Barré syndrome.
I find C. jejuni particularly intriguing to study, as surprisingly little is known about the how the bacteria behave outside of the host and the mechanisms behind the shift to a pathogenic phenotype in humans.
An area we research in our lab is the role of changes in chromosomal supercoiling – the degree to which the DNA is wound or unwound – influencing C. jejuni virulence. Pathogenic bacteria are able become more or less pathogenic by making the genes for pathogenesis sensitive to the changes in supercoiling, which are in turn influenced by environmental factors. Work carried out by Shortt and colleagues in our lab found that more supercoiled populations of C. jejuni were more mobile, while more relaxed populations were more pathogenic.
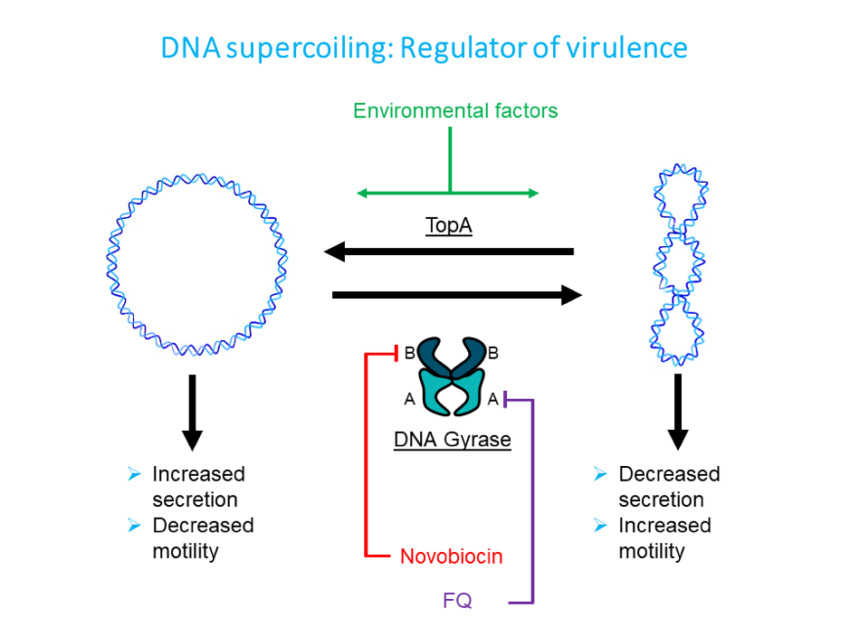
Chromosomal supercoiling is regulated by two enzymes,
TopA and DNA gyrase. They work against each other, TopA uncoiling
while DNA gyrase coils the chromosome.
The enzyme DNA gyrase is split into two subunits, A and B. In order to investigate the secretion phenotype, which is when the chromosome is more relaxed, we used novobiocin which inhibits the B subunit. By growing our C. jejuni in the presence of novobiocin, we created a population of C. jejuni with mostly relaxed chromosomes. We found that treatment with novobiocin caused a normally non-invasive strain of C. jejuni to attach to and invade epithelial cells, therefore becoming more pathogenic in humans (Scanlan et al., 2017). We found this to be a remarkable result, as these changes appear to be directly influenced by changes in chromosomal supercoiling.
During my own research into the environmental signals of supercoiling, I realised the action of novobiocin was very similar to fluoroquinolone antibiotics, which have been used both liberally in the poultry industry and as a treatment for severe Campylobacteriosis. Where novobiocin targets the B subunit of DNA gyrase, fluoroquinolones target the A subunit.
I thought to myself – what if acquisition of fluoroquinolone resistance affects DNA gyrase’s ability to cause coiling of the chromosomes, possibly causing the fluoroquinolone-resistant Campylobacter to become more virulent, as with our novobiocin model?
In work which is currently under review, we generated a panel of mutants resistant to quinolone antibiotics, including nalidixic acid and ciprofloxacin, which are used to treat severe Campylobacteriosis in humans. We (quite worryingly) found that acquisition of fluoroquinolone resistance appears to promote the ability to form biofilms, where the bacteria group together and form a protective layer that can act as a shield against antibiotics and the immune system, in conditions similar to those C. jejuni would experience on raw chicken in a supermarket environment. Our results suggest this may be caused by relaxation of the bacterial chromosome.
So far, our work suggests that the development of fluoroquinolone resistance may be associated with an increase in virulence of C. jejuni, as well as the ability to survive and form biofilms. Amazingly, this drastic behavioural change may occur through just a single mutation in the GyrA protein.
This work is of particular significance, as Campylobacter is listed as one of the World Health Organizations’ Priority Pathogens for the emergence of antibiotic resistance, specifically for fluoroquinolones. We must continue to reduce the use of such antibiotics in the agriculture industry.
The Junior Awards for Microbiology (JAM Talks) are a monthly junior seminar series aimed at integrating and connecting young researchers around Europe.
To learn more about the JAM talks, listen to our podcast with Alice Lanne and Ana Djokich, two members of the organising team, or read the blog written by Daniel Morse, who spoke at the talks in October.