Phage as tools for tackling antibiotic resistance
My name is Jessica Forsyth, I’m a research technician in bacterial evolution at the University of Manchester, UK. This work was conducted during my time as a Masters by Research student at the University of Exeter, UK under the supervision of Professor Ben Raymond and Professor Stineke Van Houte. Stineke is a phage biologist while Ben is an evolutionary biologist with a focus on applied problems, including antibiotic resistance. This work was driven by an interest in developing novel means of reducing the prevalence of antibiotic resistant bacteria. The main approach in many countries is to reduce levels of antibiotic prescribing and then to assume that antibiotic susceptible bacteria will have a better chance of outcompeting and replacing resistant genotypes. However, there are lots of reasons why a more direct approach, which aims to actively remove drug resistant bacteria from individuals could be beneficial. Active decolonization could lessen the risk of complex infection after antibiotic prescribing but has historically been used to clear out dangerous bacteria before operations such as bowel surgery.

We were particularly interested in targeted decolonization of the most harmful types of bacteria, i.e. multi-drug resistant (MDR) strains. The focus of my research was to investigate the use of lineage-specific bacteriophages in the targeted decolonisation of E. coli sequence type (ST) 131.
Extra-intestinal pathogenic E. coli (ExPEC) are of undeniable significance in the antibiotic crisis. Although able to asymptomatically colonise the gastrointestinal tract for prolonged periods of time, ExPEC can cause infection on gaining access to niches outside of the gut, such as the urinary tract or bloodstream. Infections caused by ExPEC are becoming increasingly challenging to treat owing to the emergence of resistance to first line and last-resort antibiotics such as fluroquinolones and third generation cephalosporins. ExPEC strains are the leading cause of human extraintestinal infections globally, yet only a small subset of ExPEC lineages, such as ST131, are to blame for the vast majority of infections. The targeted removal of such strains may provide us with the opportunity to achieve significant reductions in the prevalence of MDR ExPEC infections worldwide. We were interested in how this might be achieved without the use of more antibiotics.

Our research set out to investigate whether the concurrent application of phage and probiotic may present a powerful and novel interventional strategy for the eradication of MDR E. coli from the gastrointestinal tract where it is known to persist. Bacteriophages (phages) are naturally occurring viruses that infect and kill bacteria. In contrast to antibiotics, phages do not indiscriminately kill bacteria and will only target the strain of interest, meaning that disruption to the gut microbiota is minimal. Probiotics are defined as living bacteria that, when administered in adequate amounts, confer a health benefit on the host. One of the most studied probiotic strains is E. coli Nissle 1917, also known by its commercial name of ‘Mutaflor’.
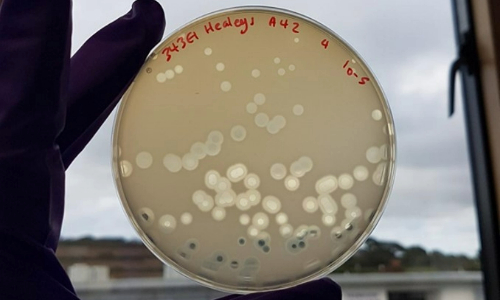
Singularly phage and probiotics are promising therapeutic agents, but both have recognised drawbacks, including the development of phage resistance. Our unique strategy aimed to combine the selection pressure imposed by phage predation with that of ecological competition that stems from the introduction of a probiotic (E. coli Nissle), to lower the frequencies of MDR strains in the gut. The benefits of this two-tiered approach are achieved via the positive interactions that exist between phage and probiotic. Although the evolution of phage resistance is almost inevitable, it is also likely to result in pleiotropic fitness trade-offs that may have a negative impact on bacterial colonisation, growth, persistence, virulence and recognition by the host immune system. Such trade-offs will enhance the capacity of the probiotic strain to effectively outcompete it and improve the efficacy of this strategy. A strategy that capitalises on the evolution of resistance as opposed to being immobilised by it is undoubtedly attractive, but its clinical implementation will rely heavily on experimental confirmation of these fitness costs and frequency dependent interactions in the body.
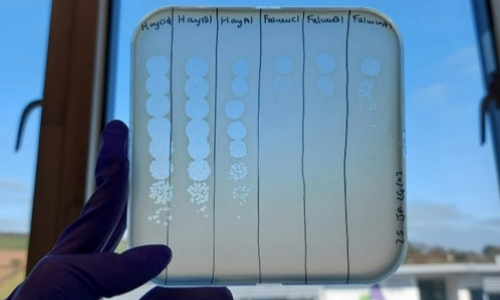
We showed that the addition of phage was able to break the frequency-dependent advantage of a numerically dominant ST131 isolate. Moreover, the addition of competing E. coli Nissle could improve the ability of phage to suppress ST131 by two orders of magnitude. Low-cost phage resistance evolved readily in these experiments and was not inhibited by the presence of a probiotic competitor. Nevertheless, combinations of phage and probiotic produced stable long-term suppression of ST131 over multiple transfers and under both aerobic and anaerobic growth conditions. Combinations of phage and probiotic therefore have real potential for accelerating the removal of drug-resistant commensal targets. Considering that the global rise in MDR infections attributed to E. coli ST131 is inextricably linked to the epidemic of the invisible and silent intestinal colonisation of ST131 in the community, the development of interventional strategies such as ours designed to target the intestinal reservoir of MDR clones are of immense importance. Removal of these commensal targets will not only act to reduce the opportunity for the development of upstream and clinically important infections such as UTI’s and bacteraemia but may also reduce the environmental spread and rates of person-to-person transmission within communities as well as the potential for the horizontal transmission of resistance genes to non-pathogenic members of the gut community.

