Toxin Tales
Posted on October 3, 2023 by Dr. Ifrah Shahi
Dr. Ifrah Shahi takes us behind the scenes of their latest publication, 'Characterization of Tigurilysin, a Novel Human CD59-Specific Cholesterol-Dependent Cytolysin, Reveals a Role for Host Specificity in Augmenting Toxin Activity' published in Microbiology.
My name is Ifrah Shahi. I was a PhD student in the laboratory of Doctor Adam Ratner at NYU Langone Health in New York City, USA. I am currently about to start a postdoctoral position as an IRACDA fellow in Doctor Joseph St. Geme’s lab at UPenn/CHOP in Philadelphia, USA.
For my work in Doctor Ratner’s lab, we looked at a well-known family of pore forming toxins called Cholesterol Dependent Cytolysins (CDCs). This family of toxins is quite large, with the majority of CDCs being produced by Gram-positive bacteria and often shown to play roles in bacterial pathogenesis. As the name suggests, all CDCs require the presence of cholesterol in the host cell membrane for successful pore formation; these ‘traditional’ CDCs are able to lyse cells from a variety of species. A small number of CDCs are also known to require an additional human host molecule for pore formation called CD59, restricting these CDCs to human cells for pore formation. By using cholesterol (and if required, human CD59) to bind to the host cell membrane, multiple CDC units can come together (i.e. oligomerize) before “punching” a hole in the host membrane. Numerous studies of CDCs and their structures, mechanisms, and functions have demonstrated how understanding the toxins in all their variations within this family of proteins provides useful insight into understanding bacterial pathogenicity itself.
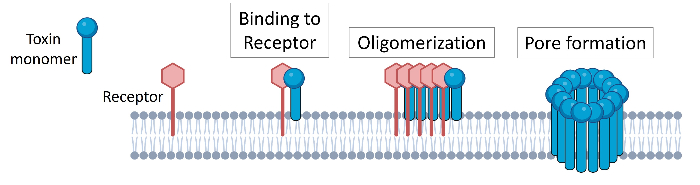
We honed in on a potential CDC produced by Streptococcus oralis subspecies tigurinus, a Gram-positive bacterial species identified fairly recently as a commensal that can cause a variety of opportunistic infections. The available genome sequence for this these bacterium included a gene suspected to code for a pore forming CDC (based on sequence similarities to other CDCs), but the protein had not previously been characterized. We purified a recombinant version of this protein and tested it for lysis of HeLa cells – only to find, disappointingly, that it had no effect. We dug further into the genome sequences of other available strains of Streptococcus oralis subspecies tigurinus and discovered a second variant of the potential CDC gene, differing from the first one by only a few amino acid residues. The recombinant version of this variant was resoundingly successful in killing HeLa cells. We named the CDC tigurilysin (TGY).
We set out to understand why the first variant of CDC was effectively non-functional in lysing cells and were able to determine that a single amino acid was the cause. This amino acid was part of a short hydrophobic loop shown in other CDCs to insert into the host membrane prior to pore formation. Our experiments also showed that this amino acid dramatically affects TGY binding to a host cell membrane – the first step of CDC pore formation – and consequently abrogates CDC oligomerization and pore formation.
We took the investigation of TGY a step further, by examining its dependence on host membrane cholesterol and CD59 for lysis. To our surprise, we found that TGY was fully dependent on both cholesterol and CD59 separately – unlike other well-known CDCs which usually display a preference for one over the other. This data, combined with the comparison of non-functional TGY with functional TGY, adds new knowledge and understanding to a fascinating field of toxin studies. Previously, structural studies comparing traditional CDCs and CD59-dependent CDCs provided valuable insight into how the protein conformations affect the host cell specificity. Given our data outlining how TGY differs from other well-characterized CDCs in its host cell specificity, it would be interesting to investigate its protein structure and how it relates to the toxin’s function.
Finally, we compared the lysis ability of a number of CDCs, both traditional and CD59-dependent, and ascertained that CD59-dependency granted CDCs a distinct advantage on HeLa cells in terms of concentration required for lysis. To more specifically look at the role of CD59-dependency in this case, we created CDC ‘hybrids’, where we swapped out host cell binding domains between CDCs. Notably, we saw that when a traditional CDC was given a host cell binding domain from a well-characterized CD59-dependent CDC, the toxin became more efficient, i.e. requiring a lower concentration to cause similar levels of cell death. The implications of these results could bring us a step closer in understanding the evolutionary advantages of human CD59-dependency in CDCs, and what that means for various CDC-producing bacterial pathogens.
Overall, increasing our knowledge of CDCs and understanding their functions and mechanisms is integral to shaping future strategies against bacterial pathogenesis. Furthermore, we now also know of a few nonpathogenic bacteria producing CDCs, and understanding these toxins is key to knowing what additional roles they might play in the microbial world.

