Understanding how common food preservatives impact Salmonella
Posted on November 12, 2025 by Prof Mark Webber
Prof Mark Webber from the Quadram Institute, UK, take us behind the scenes of their latest publication 'Common food preservatives induce an oxidative stress response in Salmonella enterica serovar Typhimurium' published in Microbiology.

My name is Prof Mark Webber based at the Quadram Institute and University of East Anglia. The Quadram Institute is interested in keeping our food safe and our group (Image 1) studies how foodborne pathogens survive in the food chain. We aim to understand this to develop improved ways to minimise food borne disease.
Salmonella is a major cause of foodborne illness around the world and can cause a range of diseases which most commonly present as gastroenteritis but can become more serious and cause life threatening systemic illness. Human infections are transmitted by contaminated food and water with a wide range of food products susceptible to becoming contaminated with Salmonella on farms or in food processing environments. Various measures to limit numbers of pathogens on food include vaccination of host animals, hygiene measures in factories, washing of produce or incorporation of preservatives in foods themselves.
Preservatives are key additions to many foods to maintain shelf lives and safety by inhibiting growth of microorganisms. A wide range of chemicals including sugars, salts, organic acids and nitrites are commonly incorporated into foods to give a preservative effect. Whilst application of preservatives is critical to keep food safe, there are concerns about some of the agents used - for example, sodium nitrite is toxic at high doses and has been associated with risk of developing cancer.
Despite the widespread use and importance of preservatives, there is relatively little known about how they act to inhibit bacterial growth, or how bacteria may adapt to preservative exposure. Given this alongside the recent calls for introduction of natural alternatives to chemical preservatives, we aimed to define how Salmonella responded to exposure to some common preservatives. This we hoped would help understand what makes a good preservative which will help in the development of new alternatives.
To explore which genes in Salmonella are involved in responses to preservatives, we used a method we have developed known as ‘TraDIS-Xpress’. In this, a massive pool of individual mutants of Salmonella were grown in the presence of four preservatives; sodium chloride, sodium nitrite, potassium chloride and sodium lactate and survival of mutants in each pool assessed.
This work showed that while there were some specific genes involved in the response to individual preservatives, there was also a core response with genes involved in central metabolism and oxidative stress responses being important for survival after exposure to all the tested preservatives (Image 2). Genes involved in creating the cell envelope were also important for some preservatives showing that barrier function is also important in dictating susceptibility for some preservatives.
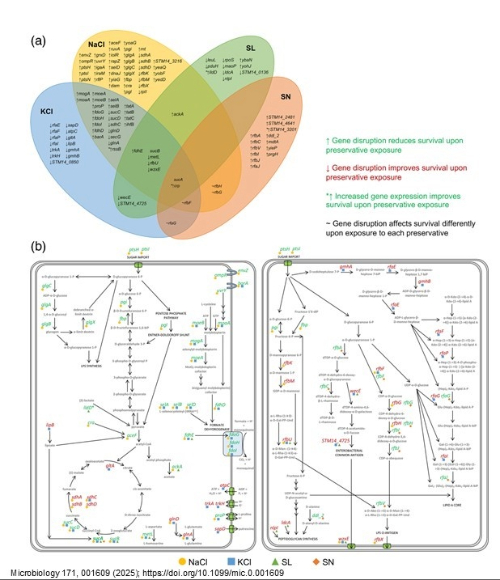
These results complemented work we had done previously where we explored how Salmonella could adapt after exposure to the same preservatives. In both studies we found signals for barrier function and oxidative stress responses being important to preservative survival.
Taken together the results from this work have shown that oxidative stress is likely to be a key mechanism by which preservatives inhibit bacterial growth and that how different preservatives enter the cell is likely to differ.
The work also identified some possible synergies between different classes of preservatives, and we hope this will inform development of new, natural preservatives by highlighting how effective preservatives act against an important food borne pathogen.
