When human drugs help fungal pathogens survive antifungal treatments
Posted on September 30, 2025 by Mariella Obermeier
Mariella Obermeier of Charité, Germany, takes us behind the scenes of their latest publication 'Non-antifungal medications administered during fungal infections drive drug tolerance and resistance in Candida albicans' published in the Journal of Medical Microbiology.
Hello! I’m Mariella Obermeier, a PhD student in the group of Prof Markus Ralser at Charité, which is a large medical school in Berlin, Germany. Together with the group of Prof Judith Berman from Tel Aviv University, Israel, we study how Candida albicans, one of the most prevalent fungal pathogens in humans, develops resistance and tolerance to antifungal treatments. One of our goals is to uncover hidden factors that undermine antifungal therapies.
Co-medications as a potential barrier to antifungal efficacy
Fungal infections are an increasing concern worldwide, particularly for patients with weakened immune systems or comorbidities requiring complex medical care. For many, antifungal drugs like fluconazole or anidulafungin are lifesaving. Yet despite careful dosing and guidelines, treatment failures still frequently occur.
Why? One underexplored reason may lie in the real-world complexity of patient medication.
Many drugs are designed to act on human cells, but since fungal cells share many similar pathways, these medications could also interact with the pathogen and affect antifungal treatment outcomes.
Reviewing common medications in high-risk patients
A major issue, however, was the lack of a systematic overview of which medications are actually taken by patients at high risk for fungal infections. To address this gap, we systematically analysed clinical (treatment) guidelines for conditions linked to high fungal infection risk. This search identified 119 (non-antifungal) drugs that are frequently given to patients who are at high risk for fungal infections across 40 medical conditions.
Finding hidden antifungal interactions with real-world relevance
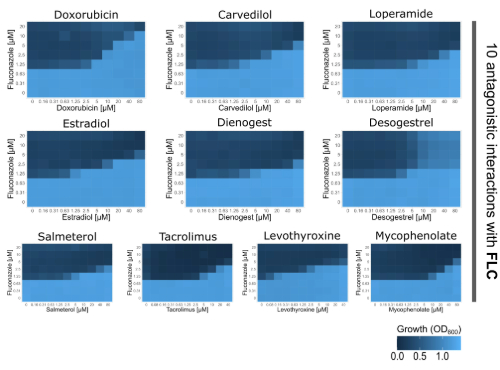
Next, we obtained all of these drugs and tested how these compounds affected C. albicans’ response to the antifungals, fluconazole and anidulafungin. Using disk diffusion and checkerboard assays, we quantified interactions, testing for synergy, indifference, or antagonism. We found that 22 of the 119 drugs – up to ~20% of all tested compounds - influenced antifungal drug responses in C. albicans, with a big majority reducing antifungal efficacy. Most drugs promoted antifungal resistance, while others increased tolerance, allowing C. albicans to continue growing under antifungal pressure. Strikingly, we also identified ten drugs that directly antagonized fluconazole by systematically increasing its minimal inhibitory concentration in a dose-dependent manner.
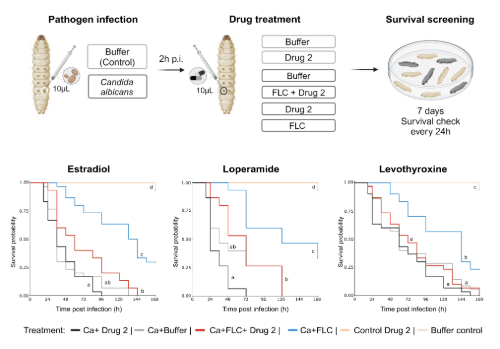
To test whether these interactions mattered beyond the lab bench, we also turned to Galleria mellonella, an established in vivo infection model. We infected G. mellonella larvae with C. albicans and treated them with fluconazole alone or combined with drugs we identified as fluconazole antagonists. We observed a clear trend: larvae treated with both fluconazole and an antagonistic drug had higher mortality than those treated with fluconazole alone, reflecting our in vitro findings.
Three combinations were particularly striking: fluconazole co-administered with estradiol (a major component of hormonal contraceptives), loperamide (an anti-diarrheal opioid agonist), or levothyroxine (a synthetic replacement for the thyroid gland hormone thyroxin T4) significantly increased larval mortality compared to fluconazole alone. This provides evidence that commonly used human medications can reduce antifungal effectiveness in vivo and should be considered as potential contributors to treatment failure.
Since we especially focused on medications commonly used in patient groups at high risk for fungal infections as part of official clinical guidelines, the antifungal–drug–pathogen combinations we study are part of the treatment of large numbers of patients.
For instance, loperamide is widely used to manage chemotherapy-related diarrhoea, a side effect affecting many patients undergoing chemotherapy. The drug is taken in extremely high doses and is freely available over the counter in most countries. Given this widespread, high-dose, and unregulated use, loperamide exemplifies how everyday medications might inadvertently compromise antifungal treatment in vulnerable patient populations.
Next steps: exploring mechanisms and clinical impact
In the short term, we hope our work will deepen our understanding of how C. albicans develops resistance and tolerance during antifungal treatment, particularly when patients are taking other medications. We are currently investigating how these drug interactions mechanistically lead to increased resistance in C. albicans during treatment.
Further, we are planning to continue to investigate the degree to which these drug interactions affect the outcome of antifungal therapies, and to which they influence the evolution of drug tolerance and resistance. This will be crucial for estimating the potential clinical impact of these interactions on antifungal treatment outcomes. In the long term, mapping these hidden interactions may help explain treatment failures, guide future clinical studies, and improve antifungal treatment guidelines.
Why this matters
Millions of people receive antifungal therapies alongside or on top of other therapies. Drug interactions are typically discussed in terms of how they affect the host. Our findings highlight the need to also consider how drug interactions influence the pathogen itself during infection treatment. While this concept has received little attention, it could help explain why some infections respond poorly to therapy.
Although our current focus is on C. albicans, similar interactions could play a role in other fungal, or potentially bacterial, infections as well. Exploring this idea further could open new avenues for understanding treatment failures and resistance development in infection biology, helping to uncover hidden factors that shape treatment outcomes.