Shortcut to a Phytoplasma Genome: Less Gear, More Discovery
My name is Zala Kogej Zwitter, and I am a PhD student at the National Institute of Biology in Slovenia, in the Department of Biotechnology and Systems Biology. My research focuses on phytoplasmas - tiny bacteria that live inside plants - and the insects that feed on them. Despite having some of the most reduced genomes among bacteria, these microorganisms can still cause devastating plant diseases, and I find that paradox fascinating. They have evolved to survive with only the bare essentials, yet their impact on agriculture can be enormous.
In Europe, one of the most serious phytoplasma diseases affects grapevines - a crop of both cultural and economic importance (and, let us be honest, we all love our wine!). In Slovenia, I have seen first-hand how growers are struggling. Flavescence dorée disease spreads rapidly, and insecticide application against its main vector, Schaphoideus titanus, remains the primary means of control. However, due to their harmful effects on bees, the most effective insecticide was banned. Growers often ask me, “What can we do now?” The honest answer is that we need more basic research before we can design better solutions. This motivated our recent paper, “From vineyard to genome: optimised enrichment and sequencing of Flavescence dorée phytoplasma from grapevine samples”, published in Microbial Genomics. Our goal was simple: to make phytoplasma genome research easier and more accessible to labs around the world.

The main challenge with phytoplasma research is that they cannot be grown in the laboratory. When you extract DNA from infected plants, you obtain mostly host DNA and only a tiny fraction of phytoplasma DNA. Standard enrichment methods exist, but they are often tedious; require specialised (and expensive) equipment, such as ultracentrifuges; or use hazardous chemicals like caesium chloride. Others rely on cultivating the pathogen in test plants such as the Madagascar periwinkle (Catharanthus roseus), which requires quarantine-level facilities when working with regulated pathogens like Flavescence dorée phytoplasma. We wanted to find a simpler, safer, and cheaper approach - something that could work directly from field samples and with equipment that most laboratories already have. Step by step, we tested different combinations to determine what was truly necessary to obtain high-quality DNA suitable for genome sequencing.
In the end, the best-performing protocol combined three key steps:
- Differential centrifugation — to remove plant cell debris using a standard centrifuge.
- CTAB extraction — a classic, reliable method for isolating DNA.
- Removal of CpG-methylated host DNA — using the NEBNext Microbiome Enrichment Kit to reduce plant DNA contamination based on methylation of eukaryotic DNA.
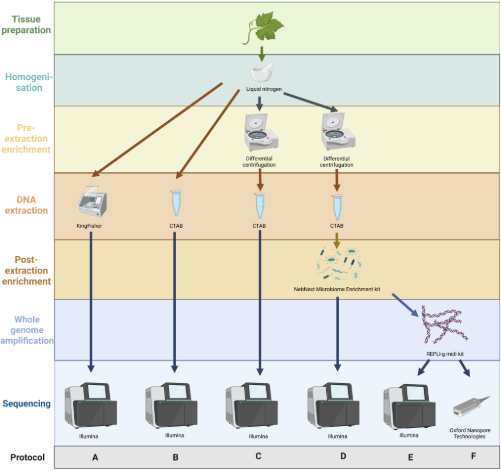
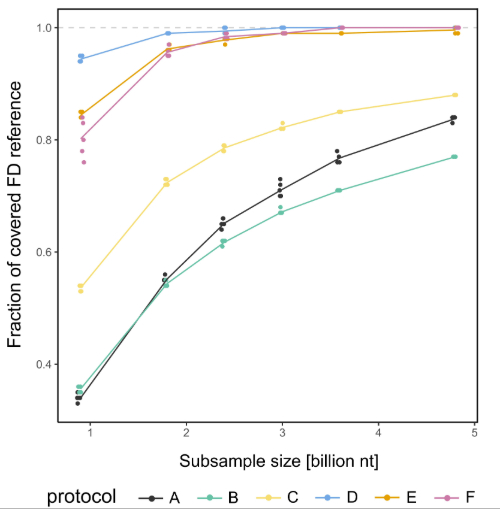
This combination significantly increased the proportion of phytoplasma DNA in our sequencing data compared to other methods. Through detailed bioinformatics analyses and subsampling tests, we found that a dataset of around 3 billion nucleotides was sufficient to cover the entire reference phytoplasma genome with read mapping. We also explored whether long-read sequencing could help us assemble these challenging genomes more completely. Using Oxford Nanopore’s MinION (a sequencer small enough to fit in your hand) we generated long reads that complemented the shorter, more accurate Illumina reads. By combining both in a hybrid assembly, we successfully reconstructed a high-quality draft genome of the Slovenian Flavescence dorée phytoplasma isolate.
But we did not stop there. To test how broadly this method could be applied, we used it on another host plant, hazelnut, infected with a related phytoplasma from the same 16SrV group. It worked remarkably well, both in leaves and roots samples. Our observations indicate that the approach has potential for broader applicability, including other phytoplasma species and possibly other non-culturable microbes. In the broader context, this protocol could help simplify phytoplasma genomics research worldwide. Improved genomic insights mean a better understanding of how these pathogens evolve, spread, and interact with their plant and insect hosts. This, in turn, enables more effective monitoring, diagnostics, and disease management strategies that are crucial for protecting crops and supporting growers.
For me, this project was not just about developing a method, but about helping a community. If more researchers can easily obtain good genomic data, the whole field can progress more rapidly. In our laboratory, we are already using this approach to compare genomes of phytoplasmas infecting hazelnut and grapevine. The hazelnut decline we observe in Slovenia (with wilting branches, yellowing leaves, and even shrub death) appears to be caused by a close relative of the Flavescence dorée phytoplasma. Understanding how similar or different they really are will help us determine how the disease spreads and, eventually, how to stop it.

Of course, knowing the pathogen is only part of the story. The next step is to deepen our understanding of the insect vectors — the tiny leafhoppers that transmit the disease from plant to plant. That is the next piece of the puzzle we are tackling. But that is a story for another day.
