From toxins to treatment: Antimicrobial proteins derived from snake venom
The Microbiology Society is undertaking a project entitled A Sustainable Future as part of our 75th Anniversary, which aims to highlight the Sustainable Development Goals (SDGs) to our members and empower them to use their research to evidence and impact the goals. Earlier this year, we put a call out to our members to submit case studies in the following three areas: antimicrobial resistance, soil health and the circular economy.
This case study is written by Dr Alasdair T. M. Hubbard, who is a Postdoctoral Research Associate and Alice J. Fraser who is a Research Assistant and at the Liverpool School of Tropical Medicine. They are both members of the Microbiology Society. It focuses on antimicrobial resistance; a naturally occurring process, whereby micro-organisms (bacteria, viruses, fungi and parasites) can change and adapt over time, either by modifying the target of the antimicrobial, or by developing and exchanging resistance genes.
Introduction to the Project
Antimicrobial resistance (AMR) is increasing globally, with low- and middle-income countries predicted to bear the biggest burden due to a myriad of socioeconomic factors, including poverty, poor sanitation, and limited access to some antimicrobials. To ensure that everyone globally has equitable access to the vital medicines to treat antimicrobial resistant infections, novel antimicrobials are desperately needed.
What are the challenges/needs that this research/initiative addresses?
There is currently a lack of economic incentives which has resulted in large pharmaceutical companies withdrawing from activity in this area. This presents an opportunity for academic institutions to fill this research space, particularly in early stage antimicrobial discovery, to find novel drug candidates for development.
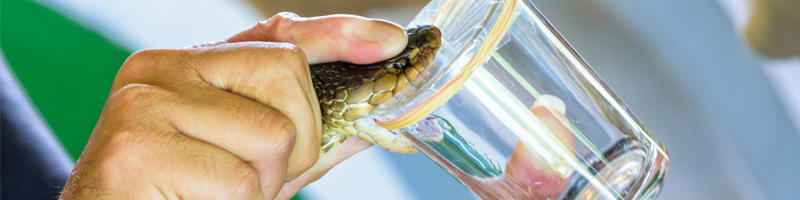
Natural products represent an attractive source for antimicrobial discovery and are often underexplored. Several studies have highlighted the antimicrobial activity of a variety of different venoms, including from snakes, centipedes, bees, scorpions, and spiders. Venoms contain a wide range of different proteins, although studies into their antibacterial activity often focus on individual proteins which are easily purified and are not developed any further.
At the Liverpool School of Tropical Medicine (LSTM), we are in a position to investigate the antibacterial activity of venom further; the collaboration between the Centre for Snakebite Intervention and Research at LSTM, which houses the largest collection of venomous snakes in the UK, the Centre for Drugs and Diagnostic which has extensive experience of drug development, and the AMR research group represents a unique opportunity and resource for early stage antimicrobial discovery projects.
What findings and solutions were provided by this research/initiative?
During our research project we have developed a simple, high throughput assay to screen whole snake venom for antibacterial activity towards Escherichia coli. Any snake venom which demonstrated antibacterial activity can then be separated into the different protein classes and iteratively tested to try and identify the protein classes responsible for the antibacterial activity. So far, we have identified five classes of protein with antibacterial activity from elapid snakes (a major family of snakes which includes the cobras and mambas). These proteins can then be further purified to confirm their antibacterial activity before entering the antimicrobial development pipeline, as well as determining their mode of action in order to identify other inhibitors which could also be developed into antimicrobials. As we have been able to demonstrate success using this assay, it is now being expanded to include a larger range of snake venom, venom from other venomous animals and against a wider selection of pathogenic bacteria. Not only does composition of venom differ between the different venomous animals, genus and species but also, in some animals, can depend on the location, age and diet of a single species. Consequently, there is an almost inexhaustive number of different venoms to screen for antibacterial activity and mine for novel antimicrobials.
Although antimicrobial discovery and development is certainly a difficult and long process, there are undoubtedly more natural products out there which can be developed into antimicrobials waiting to be discovered. With this assay we also aim to speed up the process of antimicrobial discovery from venomous animals by quickly and easily screen a large number of venoms to identify the proteins present with antibacterial activity, thereby increasing the chances of finding candidate(s) that can enter the antimicrobial development pipeline.
What is the future for research and innovation in this area?
This project has underlined that more early stage antimicrobial development research programmes are desperately needed to meet the increasing clinical demand for novel antimicrobials to treat future antimicrobial resistant infections. However, early stage drug development is only one way of mitigating the threat that AMR poses and must be used in conjunction with other strategies. These include improved diagnostics, the development of new treatment strategies to combat emerging antimicrobial resistance developing in the first place and increase public awareness through educational campaigns. We have used this project to capture public attention as snake venom is an exotic and unexpected source to find novel antimicrobials. Using this platform, we have been able to simultaneously engage and educate the public about the threats of antimicrobial resistance and explain how our research at the LSTM is addressing this pressing global public health concern.
With the antimicrobial drug discovery and development research gap widening, it will be remiss of scientists, particularly microbiologists, biochemists, and medicinal chemists, not to fill this space to ensure the development pipeline does not grind to a halt. However, it is important to remember that combatting antimicrobial resistance is not just the responsibility of scientists and healthcare professionals, everyone has a role to play.


About the authors
Dr Alasdair T. M. Hubbard is a Postdoctoral Research Associate and Alice J. Fraser is a Research Assistant and at the Liverpool School of Tropical Medicine.
More information about their work is available here.


