Making antibiotics work better

The Microbiology Society is undertaking a project entitled A Sustainable Future as part of our 75th Anniversary, which aims to highlight the Sustainable Development Goals (SDGs) to our members and empower them to use their research to evidence and impact the goals. Earlier this year, we put a call out to our members to submit case studies in the following three areas: antimicrobial resistance, soil health and the circular economy.
This case study is written by Dr Andrew Edwards, who is a Senior Lecturer in Molecular Microbiology at Imperial College London, and a member of the Microbiology Society. It focuses on antimicrobial resistance; a naturally occurring process, whereby micro-organisms (bacteria, viruses, fungi and parasites) can change and adapt over time, either by modifying the target of the antimicrobial, or by developing and exchanging resistance genes.
Introduction
Antibiotics are essential for many areas of modern medicine enabling surgery, organ transplantation, cancer chemotherapy and the survival of pre-term infants, people with cystic fibrosis. and those with compromised immune systems. All of this is threatened by a combination of antibiotic resistant infectious bacteria and a lack of new antibiotics in the development pipeline.
What are the challenges that this research addresses?
In addition to the many technical challenges associated with antibacterial drug discovery, the major hurdle is the poor economic return on new antibiotics. Unlike most new drugs, new antibiotics are used as sparingly as possible to reduce the emergence of resistance. This limits sales and thus revenue for the companies concerned. Consequently, many large and medium pharmaceutical companies have withdrawn from antibiotic research and development.
Therefore, the challenge is to develop new anti-bacterial approaches that can be used widely, thus benefitting from a larger market, without selecting for resistance.
What findings and solutions were provided by this research?
During our research, we identified a single target in bacteria, which if blocked would be expected to sensitise the organism to multiple different classes of antibiotics. Not only did this increase the anti-bacterial activity of the antibiotics, it also re-sensitised drug-resistant bacteria to some antibiotics. Therefore, inhibitors of this bacterial target could be used with multiple different existing antibiotics to enhance treatment outcomes, even against resistant bacteria. In addition to sensitising bacteria to antibiotics, inhibition of this new target also sensitised bacteria to killing by host immune cells, providing a second mechanism by which this approach could help clear hard to treat infections. Finally, we also found that inhibition of the identified target reduces the mutation rate, slowing the emergence of mutants resistant to antibiotics.
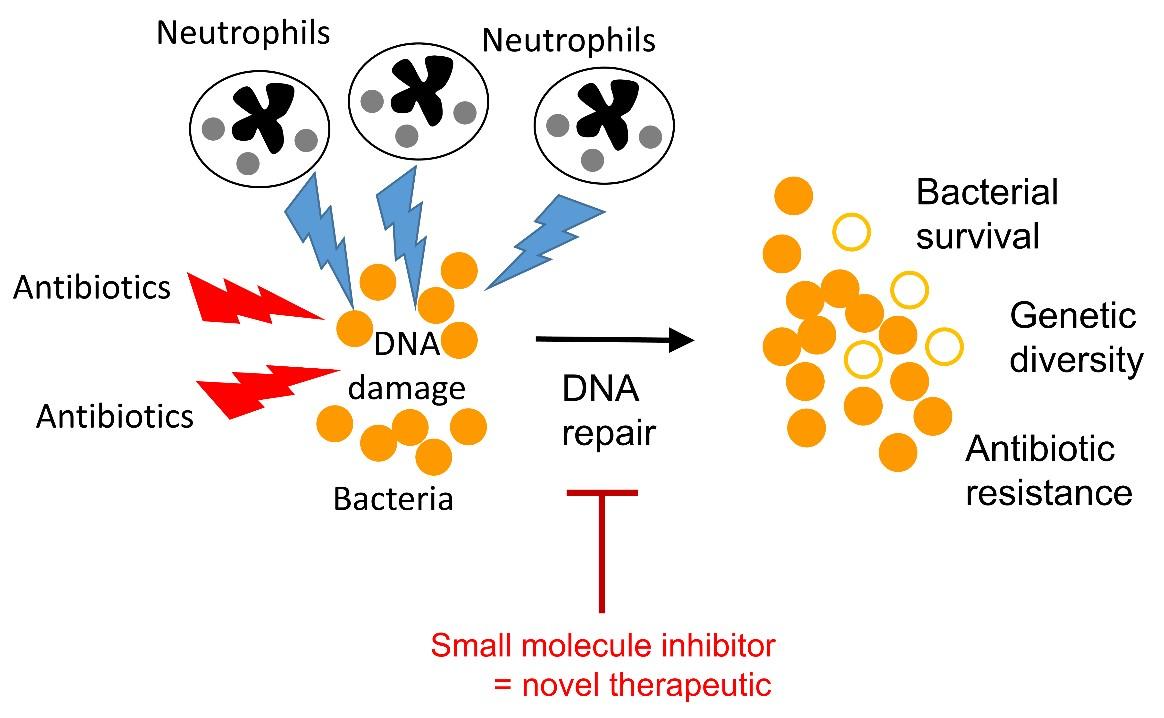
Working with colleagues in the Department of Chemistry at Imperial College London, we have started to develop small molecule inhibitors of our target, which have been shown to reverse resistance to quinolone antibiotics in MRSA. Ongoing library screens and compound optimisation is expected to yield even better high-affinity inhibitors, that could be used to sensitise or re-sensitise a broad range of different bacteria to antibiotics and host defences.
How can this research support the transition to a more sustainable future?
The development of new antibacterial drugs will enable the continuation of surgery, cancer chemotherapy and survival of those with compromised immune systems. By developing a broad-spectrum approach that works with several different antibiotics, as well as sensitising bacteria to immune defences; this work provides a potential route to multiple new treatments for multiple types of infection. In addition, we hope that this approach will slow the rate of emergence of resistance to existing antibiotics.
What is the future for research and innovation in this area?
There are many new approaches to antibacterial therapeutics that have shown promise in early studies including novel antibiotics, the use bacteriophages, monoclonal antibodies and antimicrobial peptides. The challenge is to attract the funding and other support needed to develop these into clinical applications. There remains a large gulf between what industry wants to see in terms of proof of concept and what academia can deliver. Closing this gap requires substantial investment and a complete overhaul of financial incentives for pharmaceutical companies to invest in antibacterial therapeutics.
About the author

Dr Andrew Edwards is a Senior Lecturer in Molecular Microbiology at Imperial College London and a member of the Microbiology Society. More information about his work is available here.


