From the recovery of copper from malt whisky distillery wastewater to an integrated system for distillery waste management and minimisation

The Microbiology Society is undertaking a project entitled A Sustainable Future as part of our 75th Anniversary, which aims to highlight the Sustainable Development Goals (SDGs) to our members and empower them to use their research to evidence and impact the goals. Earlier this year, we put a call out to our members to submit case studies in the following three areas: antimicrobial resistance, soil health and the circular economy.
This case study is written by Dr Beate Christgen, Professor Ian Head, Professor Eileen Yu and Dr Ronald Daalmans, as part of their work on the Microbial Electrochemical Technology for Resource Recovery (MeteoRR) project. It focuses on the Circular Economy; an alternative to a traditional linear economy (make, use, dispose), in which we keep resources in use for as long as possible, extract the maximum value from them while in use, then recover and regenerate products and materials at the end of each service life.
A growing human population and global economic development are driving increased demand for mineral and energy resources. Modern electronic devices depend on both rare metals like lanthanides and more common, but nonetheless important, metals such as copper. To satisfy our need for smartphones and other technologies, metal scarcity is a growing problem. While one solution to mineral scarcity is end-of-use recycling of electronic devices, most metals are still extracted from geological primary ore deposits. This depletes finite resources and leads to increasing amounts of waste containing critical minerals.
Copper is an essential metal for modern society and has many uses in electronics. A desktop computer may contain as much as 1.5 kg of copper and a smartphone contains around 15g of copper. Global demand for copper is projected to increase from 213% to 341% by 2050, and it is estimated that demand for copper from geological sources will peak around 2025-2030 (Elshkaki et al., 2018). It is therefore paramount that more sustainable routes to copper production should be explored.
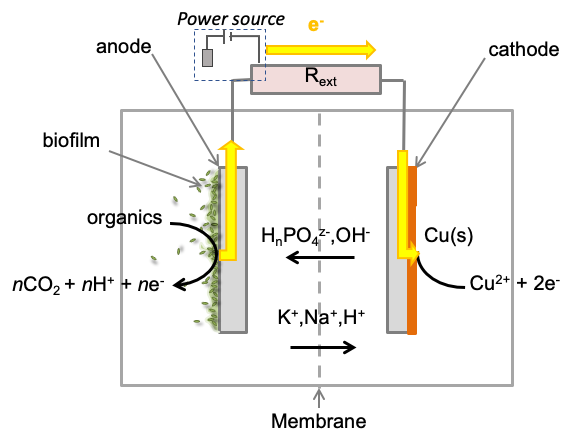
Figure 1: The operation of BES for metal recovery. Metals with a sufficiently positive reduction potential (including copper) can be reduced directly in a BES fuelled solely by oxidation of organic compounds on the anode with no additional energy input required. A small additional applied voltage can enhance metal recovery and allow reduction of metals with more negative reduction potential such as Ni or Zn.
One way to reduce reliance on primary ores for copper is to consider waste as a resource for copper recovery. While copper is an essential element in many biological systems, in the environment copper is a toxic metal and shows a toxic effect towards a wide range of organisms, in some cases, even at micromolar levels (Sanchez et al., 2007). Removal of toxic metals from waste is thus important from both an environmental perspective, but simultaneous mitigation of metal pollution and recovery of a valuable resource in a circular economy-like model potentially offers both environmental and economic benefits.
Industrial, municipal and agricultural wastewaters are potential sources of metals and chemical salts, acids and bases, and energy. Efficient exploitation of these waste streams for metal recovery depends on understanding the underlying chemical, electrochemical and biological processes dictating the behaviour of metals in a waste stream. Metal containing wastes often have low metal concentrations (micrograms to milligrams/L). This can be technically difficult for conventional recovery methods and is not economically viable.
Bioelectrochemical systems (BES) can overcome some of the limitations of conventional metal recovery technologies. In their simplest form, BES consist of an anode, a cathode, and a microbial catalyst associated with the anode, the cathode or both. Bacteria on the anode, transfer electrons, from oxidation of organic electron donors such as organic compounds in wastewater, to the anode and through an external circuit to the cathode to produce electricity (a microbial fuel cell). In microbial fuel cells the electrons at the cathode are used to reduce oxygen to water, but they can also be used to selectively reduce, and therefore recover metal ions from waste streams (Figure 1; Christgen et al., 2020) or synthesise valuable chemical feedstocks such as organic acids and alcohols from CO2 reduction in a process often referred to as microbial electrosynthesis (Fortmorin et al., 2019; Izadi et al., 2020).

Figure 2: Copper deposition on a graphite plate cathode.
There are many industrial processes that produce waste streams containing copper. Our work has focused on malt whisky production. Scotch whisky production is a multibillion-pound industry and accounts for £4 billion of exports from the UK. Malt whisky production specifically has at its heart the use of pot stills made from copper. The use of copper is an essential part of the process, as it removes sulphur compounds that affect the aroma and taste of the produced whisky. Spent lees, the effluent from the second distillation has a high organics content and a copper concentration of 10–50 mg/l and, as a medium sized distillery, produces around 388,000L spent lees per week, this equates to close to half a ton of copper released annually from each medium sized malt whisky distillery.
We developed a system for copper recovery from spent lees using BES. BES reactors and electrochemical half cells with graphite electrodes were used to test copper recovery and deposition from spent lees from different distilleries. High copper deposition on graphite electrodes was observed (Figure 2) with over 50mg of copper being deposited from a litre of spent lees over a 48-hour period, while reducing the copper concentration in solution to less than 1 mg/l. When a small external voltage (0.5V) was applied to the BES, pure copper metal was recovered on the cathode. Even with no external energy input, copper was still effectively removed from spent lees, but cuprite (Cu2O) was the main deposit on the electrode with a smaller amount of copper metal. Controlling the cathode potential by altering the external resistance on the BES, allowed the system to be tuned either for copper metal recovery or deposition of cuprite (Cu2O) which can be used as a catalyst for CO2 reduction to formate and CO, useful chemical feedstocks (Uemoto, N. et al., 2019; Xiang, H. et al., 2019). Altogether, copper recovery from spent lees would make a positive impact both economically and environmentally.
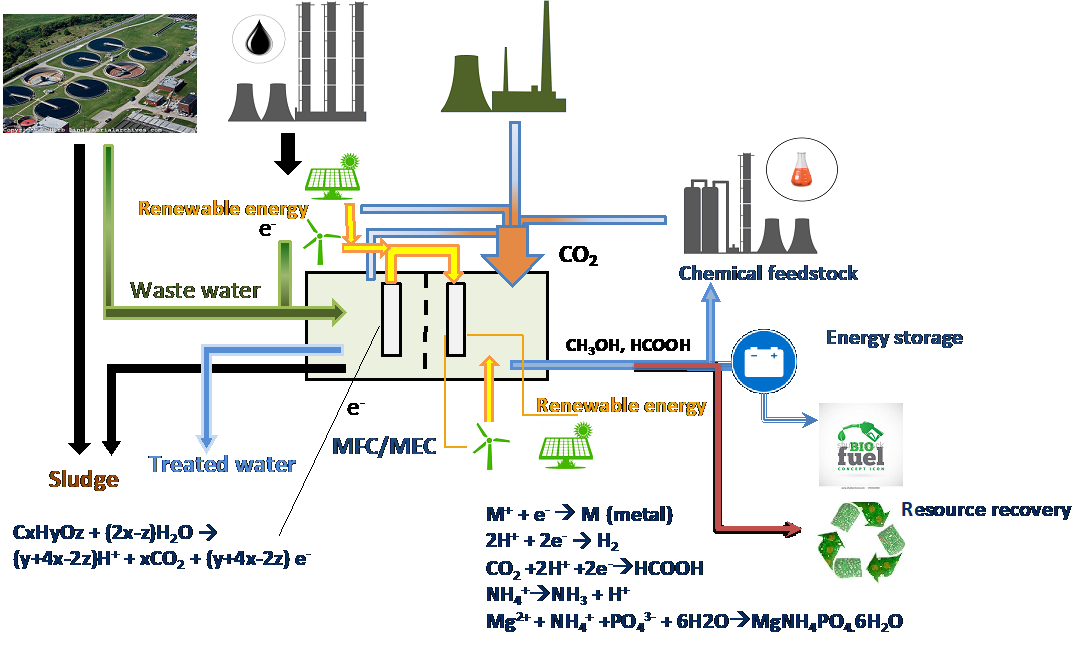
Figure 3: Integrated system for distillery waste management and minimisation.
Based on encouraging lab scale results (Kaur et al., 2019) and a life cycle assessment of the process (Sadhukhan et al., 2016), we are exploring the potential for an integrated system for distillery waste management and minimisation towards achieving a circular economy for malt whisky production. This system (Figure 3) integrates organic carbon removal from distillery waste for energy production to recover copper, recovery of low waste heat to run the BES system at a stable temperature, convert waste CO2 to valuable chemicals and, at the same time, treat the distillery wastewater.
References
Christgen, B., Suarez, A., Milner, E., Boghani, H., Sadhukhan, J., Shemfe, M., Gadkari, S., Kimber, R.L., Lloyd, J.R., Rabaey, K., Feng, Y., Premier, G.C., Curtis, T., Scott, K., Yu, E. and Head, I.M. (2020) 'Metal Recovery Using Microbial Electrochemical Technologies', in Resource Recovery from Wastes: Towards a Circular Economy. The Royal Society of Chemistry, pp. 87-112.
Elshkaki, A., Graedel, T.E., Ciacci, L. and Reck, B.K. (2018) 'Resource Demand Scenarios for the Major Metals', Environ Sci Technol, 52(5), pp. 2491-2497.
Fontmorin, J.-M., Izadi, P., Rasul, S., and Yu, E.H. (2019). Carbon dioxide utilisation by bioelectrochemical systems through microbial electrochemical synthesis. in Transformations, edited by North, M. and Styring, P. (2019) De Gruyter, Germany, pp. 561-581.
Izadi, P., Fontmorin, J-M, Godain, A., Yu, E., Head, I. (2020). Parameters influencing the development of highly conductive and efficient biofilm during microbial electrosynthesis: the importance of applied potential and inorganic carbon source. npj Biofilms and Microbiomes in press
Kaur, A., Boghani, H.C., Milner, E.M., Kimber, R.L., Michie, I.S., Daalmans, R., Dinsdale, R.M., Guwy, A.J., Head, I.M., Lloyd, J.R., Yu, E.H., Sadhukhan, J. and Premier, G.C. (2019) 'Bioelectrochemical treatment and recovery of copper from distillery waste effluents using power and voltage control strategies', Journal of Hazardous Materials, 371, pp. 18-26.
Sadhukhan, J., Lloyd, J.R., Scott, K., Premier, G.C., Yu, E.H., Curtis, T. and Head, I.M. (2016) 'A critical review of integration analysis of microbial electrosynthesis (MES) systems with waste biorefineries for the production of biofuel and chemical from reuse of CO2', Renewable and Sustainable Energy Reviews, 56, pp. 116-132.
Sanchez, D., Graca, M.A.S. and Canhoto, J. (2007) 'Testing the use of the water milfoil (Myriophyllum spicatum l.) in laboratory toxicity assays', Bulletin of Environmental Contamination and Toxicology, 78(6), pp. 421-426.
Uemoto, Naoki; Furukawa, Mai; Tateishi, Ikki; Katsumata, Hideyuki; Kaneco, Satoshi. (2019). ‘Electrochemical Carbon Dioxide Reduction in Methanol at Cu and Cu2O-Deposited Carbon Black Electrodes.’ ChemEngineering 3, 3(1), p.15. Available at: http://dx.doi.org/10.3390/chemengineering3010015
Xiang, H., et al., Enhanced selectivity of carbonaceous products from electrochemical reduction of CO2 in aqueous media. Journal of CO2 Utilization, 2019. 30: p. 214-221
About the Authors
Dr Beate Christgen is a Senior Researcher in Geomicrobiology and Professor Ian Head is a Professor in Environmental Microbiology at the School of Natural and Environmental Sciences at Newcastle University, UK and a member of the Microbiology Society. Professor Eileen Yu is Professor of Electrochemical Engineering at Loughborough University, UK. Dr Ronald Daalmans is Environmental Sustainability Manager at Chivas Brothers. More information about their work on resource recovery from wastes can be found here (www.meteoRR.ac.uk).
The work presented here was part of the Microbial Electrochemical Technology for Resource Recovery (MeteoRR) project, a collaboration led by the University of Newcastle with collaborators in the Universities of South Wales, Manchester and Surrey, with a range of industry partners.
Funding
NERC Microbial Electrochemical Technology for Resource Recovery (MeteoRR) project (www.meteoRR.ac.uk, NE/L01422X/1) of the Resource Recovery from Waste program (https://nerc.ukri.org/research/funded/programmes/waste/) and the EPSRC Liquid Fuels & Energy supply from CO2 Reduction (LifesCO2R) project (https://research.ncl.ac.uk/lifesco2r/; EP/N009746/1)


